Cancer Immunotherapy: Viruses, Vaccines, and other Immuno-Oncology Treatments
Blog post by Jasmin Skinner
Despite significant advances in chemotherapy, radiation therapy, and surgical options, cancer remains the second leading cause of death in the United States (1). Consequently, there remains a pressing need for new and effective methods of cancer prevention and treatment. In recent years, vaccines have emerged as a promising approach for both preventing the development of cancer and treating established malignancies (1). In this article, we will explore the use of vaccines for the prevention and treatment of cancer, highlighting a number of existing therapies and discussing the potential clinical use of cancer vaccines.
This webinar features a discussion of current and emerging methods, recent publications, and future directions for the field of cancer immunotherapy. Participants will gain an understanding of how the cancer immunity cycle impacts cancer immunotherapy development, and will also learn about a few novel solutions that could help overcome the difficulties of immunotherapeutic development. WATCH NOW
Prophylactic Cancer Vaccines
Prophylactic, or preventive, vaccines are designed to prevent the onset of cancer by eliciting a specific immune response against cancer cells before they can develop (1). One well-known example is the human papillomavirus (HPV) vaccine, which targets a sexually transmitted virus that commonly causes cervical and oral cancer (1). The HPV vaccine acts by stimulating the immune system to produce neutralizing antibodies that prevent the HPV virus from binding to a host cell, thereby preventing the development of cancer (1).
Another example of a prophylactic cancer vaccine is the hepatitis B virus (HBV) vaccine. HBV is a leading cause of hepatocellular, or liver, cancer globally, and the HBV vaccine has been shown to be highly effective in preventing HBV infection and reducing the risk of liver cancer (1). HBV vaccinations of infants in Taiwan have shown that the rate of hepatocellular cancer in vaccinated children aged 6–14 was reduced by approximately 70% (1).
Therapeutic Cancer Vaccines
In contrast, therapeutic vaccines are administered after a patient has received a cancer diagnosis. Their intent is to reduce and eliminate any remaining tumor cells while also establishing a persistent anti-tumor immune memory for long-term protection against cancer recurrence (2). The underlying principle of therapeutic vaccines is to prime the immune system against tumor-specific antigens, allowing the body to mount an appropriate immune response (2). There are currently three major types of therapeutic cancer vaccines: viral, cellular, and peptide-based vaccines (3).
Therapeutic viral vaccines affect tumor prevention and eradication with a notably different mechanism of action when compared to their preventive counterparts. While prophylactic viral vaccines prime the immune system against tumor-specific (TSA) and tumor-associated antigens (TAA) prior to diagnosis, therapeutic viral vaccines contain an oncolytic virus which selectively infects and destroys cancer cells while sparing healthy tissue (4). These vaccines have been shown to improve the immune system’s ability to kill tumor cells, likely due to the release of tumor antigens following oncolysis (4). Viruses are also used therapeutically in viral vector vaccines, which contain a virus encoded with TAA’s. Like oncolytic viral vaccines, these are also typically modified to reduce their ability to infect and replicate within healthy cells (5).
Cellular vaccines are classified into two major groups, depending on the method of development. Autologous vaccines are derived from a patient’s own tissues, whereas allogeneic vaccines are derived from the tumor cells of a different patient (3). Both of these methods are capable of producing dendritic cell vaccines, the very first therapeutic cancer vaccine approved by the US Food and Drug Administration (FDA) (6). This method of vaccination utilizes these cells’ ability to mediate interactions between foreign bacteria and the immune system (7).
In a healthy patient, immature dendritic cells sample, capture, and internalize antigens from the external environment. They then migrate to the draining lymph nodes and present processed antigens to T cells, allowing them to mount an appropriate immune response. Dendritic cell vaccines use this same principle by administering TAA-loaded dendritic cells, such that they may migrate to nearby lymph nodes and trigger an immune response against the cancer cells (7).
Peptide-based vaccines use an amino acid sequence derived from either TAA or TSA’s 8). As the name implies, tumor-specific antigens are found only within tumors, whereas tumor-associated antigens are found on both healthy and cancerous cells, but are far more elevated in the latter (8).
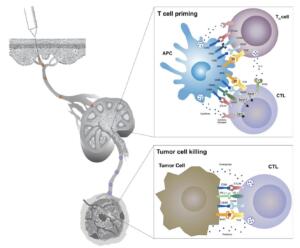
Figure 1: Mechanism of T cell activation and T cell-mediated cancer cell destruction. Cytotoxic T lymphocytes (CTLs) are primed when a T cell receptor (TCR) on their surface binds to an MHC-antigen complex displayed on an antigen presenting cell (APC). This triggers a signaling cascade resulting in T cell activation and proliferation, migration to the tumor tissue, and ultimately elimination of cancer cells diplaying that antigen. © 2019 Hollingsworth & Jansen, licensed under CC BY 4.0.
Clinical Trials and the Prospects of Cancer Vaccines
Clinical trials are ongoing to assess the efficacy and safety of vaccines for both preventing and treating cancer. To date, these trials have produced promising results, with several vaccines demonstrating efficacy in the treatment of melanoma, prostate cancer, and leukemia (5).
One illustrative example of a successful therapeutic cancer vaccine is sipuleucel-T, which was approved by the FDA in 2010 for the treatment of metastatic prostate cancer (6). Sipuleucel-T is a dendritic cell vaccine that uses a patient’s own dendritic cells, which have been modified to present prostate cancer antigens. In clinical trials, sipuleucel-T was shown to increase overall survival in patients, with 38% increase in survival after 3 years (6).
Another promising candidate is the talimogene laherparepvec (T-VEC) vaccine, which was approved by the FDA in 2015 for the treatment of advanced melanoma (Pol). T-VEC is a viral vaccine that utilizes a modified herpes simplex virus to infect and lyse cells, subsequently releasing TSA’s into the extracellular environment which will ultimately promote an anti-tumor immune response (5). Phase I & II clinical trials showed promising results in patients with various types of tumors, including melanoma (9). Phase III trials further support this as T-VEC resulted in a 4 month increase in overall survival when compared to a control treatment (5).
In addition to these vaccines, several other promising therapeutic cancer vaccines are currently in development. For example, researchers are investigating the use of peptide vaccines for the treatment of glioblastoma, a highly aggressive form of brain cancer. However, a lack of specificity and high expression of epitopes have somewhat limited development of peptide-based vaccines for this application (10).
Nevertheless, the development of personalized cancer vaccines is promising, and involves tailoring treatment to the unique heterogeneity of a patient’s tumor (8). It is hoped that these vaccines will reduce some of the unwanted characteristics of standardized peptide vaccines, and they may yet prove particularly effective in patients who have not responded to traditional therapy (8).
Implications and Future Directions
The use of vaccines for the prevention and treatment of cancer represents a promising area of research that has the potential to revolutionize cancer therapy. While several prophylactic vaccines have been developed to prevent cancers caused by specific viruses, such as HPV and HBV, therapeutic vaccines offer an exciting new approach to the treatment of established malignancies (1,2). Early indications are encouraging, as clinical trials have shown that therapeutic vaccines, such as sipuleucel-T and T-VEC, can increase overall survival in patients with metastatic prostate cancer and advanced melanoma, respectively (6,9).
While much work remains to be done to fully understand the potential of vaccines to prevent and treat cancer, recent advances in cancer immunotherapy provide cause for optimism for patients and researchers alike. By continuing to explore the potential of vaccines and to refine our understanding of the immune system’s role in cancer, new and effective treatments to significantly improve outcomes may be on the horizon.
About the Author
About the Author

Jasmin Skinner is an undergraduate student at the University of Western Ontario completing a Specialization in Biology and a Minor in Chemistry, with focused interest in applying these concepts to environmental conservation. As a lover of the outdoors and the arts, much of her time is spent in nature and within the local London art community, creating and connecting with all walks of life. After graduating, she hopes to continue her passion of finding unconventional solutions to environmental issues by working with nature, not against it.
References
- Crews DW, Dombroski JA, King MR. Prophylactic cancer vaccines engineered to elicit specific adaptive immune response. Front Oncol [Internet]. 2021 Mar [cited 2023 March];11:626463. Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC8044825/. doi: 10.3389/fonc.2021.626463
- Saxena M, van der Burg SH, Melief CJM, Bhardwaj N. Therapeutic cancer vaccines. Nat Rev Cancer [Internet]. 2021 April [cited 2023 March]; 21:360-78. Available from: https://www.nature.com/articles/s41568-021-00346-0. doi: 10.1038/s41568-021-00346-0
- Hollingsworth RE, Jansen K. Turning the corner on therapeutic cancer vaccines.
NPJ Vaccines [Internet]. 2019 Feburary [cited 2023 March]; 4:7. Available from: https://www.nature.com/articles/s41541-019-0103-y#:~:text=In%20general%2C%20three%20types%20of,peptides%2C%20DNA%2C%20or%20RNA. doi: 10.1038/s41541-019-0103-y - Larocca C, Schlom J. Viral vector-based therapeutic cancer vaccines. Cancer J [Internet]. 2011 September [cited 2023 March];17(5):359-71. Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3207353/ doi: 10.1097/PPO.0b013e3182325e63
- Kamta J, Chaar M, Ande A, Altomare DA, Ait-Oudhia S. Advancing cancer therapy with present and emerging immuno-oncology approaches. Front Oncol [Internet]. 2017 April [cited 2023 March]; 7:64. Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5394116/. doi: 10.3389/fonc.2017.00064
- Cheever MA, Higano CS. PROVENGE (Sipuleucel-T) in prostate cancer: the first FDA-approved therapeutic cancer vaccine. Clin Cancer Res [Internet]. 2011 June [cited 2023 March]; 17(11):3520-6. Available from: https://aacrjournals.org/clincancerres/article/17/11/3520/12151/PROVENGE-Sipuleucel-T-in-Prostate-Cancer-The-First. doi: 10.1158/1078-0432.CCR-10-3126
- Datsi A, Sorg RV. Dendritic cell vaccination of glioblastoma: road to success or dead end. Front Immunol [Internet]. 2021 November [cited 2023 March]; 12:770390. Available from: https://www.frontiersin.org/articles/10.3389/fimmu.2021.770390/full#:~:text=Dendritic%20cell%20vaccination%20(DCV)%20is,and%20confirming%20feasibility%20and%20safety. doi: 10.3389/fimmu.2021.770390
- Stephens AJ, Burgess-Brown NA, Jiang S. Beyond just peptide antigens: the complex world of peptide-based cancer vaccines. Front Immunol [Internet]. 2021 June [cited 2023 March]; 12:696791. Available from: https://www.frontiersin.org/articles/10.3389/fimmu.2021.696791/full. doi: 10.3389/fimmu.2021.696791
- Pol J, Kroemer G, Galluzzi L. First oncolytic virus approved for melanoma immunotherapy. Oncoimmunology [Internet]. 2015 December [cited 2023 March]; 5(1): e1115641. Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4760283/#:~:text=On%202015%2C%20October%2027th%2C%20the,the%20skin%20and%20lymph%20nodes. doi: 10.1080/2162402X.2015.1115641
9 - Zhao T, Li C, Ge H, Lin Y, Kang D. Glioblastoma vaccine tumor therapy research progress. Chin Neurosurg J [Internet]. 2022 January [cited 2023 March]; 8:2. Available from: https://cnjournal.biomedcentral.com/articles/10.1186/s41016-021-00269-7. doi: 10.1186/s41016-021-00269-7
