Clearing the Brain Fog: Long COVID and Cognitive Impairment
Blog post by Andrew Davies, PhD
The COVID-19 pandemic is now approaching its third year, with more than 500 million severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) cases reported globally. Accompanying the ever-increasing number of COVID survivors is a greater appreciation of the long-term consequences of infection. One frequently reported post-recovery symptom is ongoing cognitive impairment, often referred to as “COVID fog” or brain fog, which may affect up to 25% of recovered individuals [1].
Although most commonly reported following severe cases, COVID fog is also relatively common among those recovered from mild initial symptoms. In addition to impaired concentration and memory, long COVID has also been associated with increased depression, anxiety, fatigue, and disrupted sleep patterns [2]. Given the global prevalence of SARS-CoV-2 and the detrimental impact of such neurological and cognitive impairment, the public health implications are widespread and considerable. In a recent paper published in Cell, Fernández-Castañeda et al. examined the underlying neurological changes associated with COVID fog (Figure 1) [3], which we briefly touched on in a recent blog post, and review more extensively here.
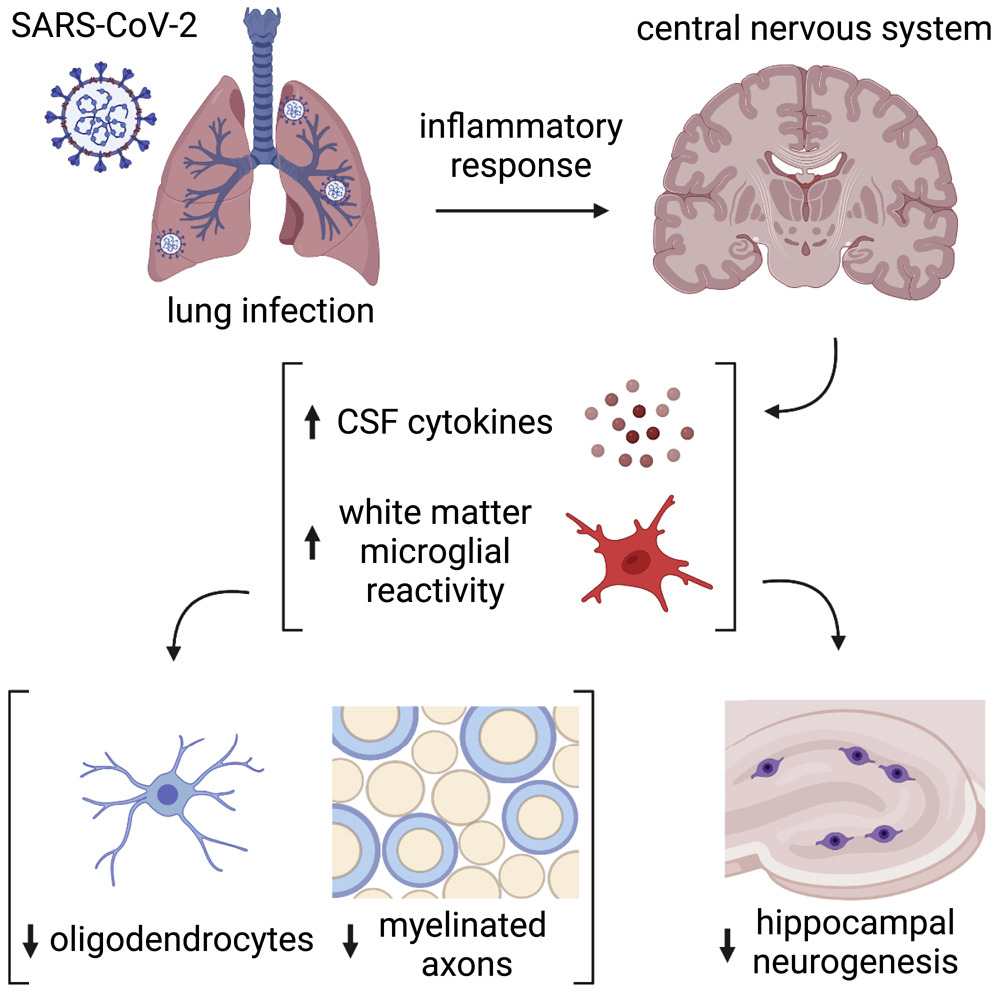
Figure 1: Schematic illustration of the neurobiological effects of respiratory SARS-CoV-2 infection. © 2022 Fernández-Castañeda et al., licensed under CC BY 4.0.
COVID fog and chemo fog: a possible clue?
As noted by the authors, the syndrome of cognitive symptoms observed in long COVID bears some resemblance to that observed in patients undergoing certain cancer therapies, leading to their hypothesis that the underlying pathology may also be similar. Notably, microglial reactivity may be affected by some chemotherapy treatments and radiation, resulting in elevated central nervous system cytokine levels, impaired neuroplasticity and neurogenesis, and inflammatory neurotoxicity. They therefore sought to determine whether similar changes in microglial activity and cytokine levels were observed following SARS-CoV-2 infection.
Neuroinflammation and SARS-CoV-2
The authors report that SARS-CoV-2 infection resulted in an inflammatory response in excess of that typically seen with other common viral infections of the respiratory system (e.g., H1N1 influenza). The observed changes in microglial reactivity persisted following COVID, particularly in white matter regions (Figure 2), and elevated levels of the chemokine CCL11 were reported in both animal models and human subjects experiencing COVID fog. Fernández-Castañeda et al. suggest that the observed selective susceptibility of certain brain regions (e.g., the hippocampus) to elevated levels of CCL11 may at least partly account for differences in the neurological impact of certain viral infections. Loss of oligodendrocytes and myelin were also evident in mouse models of SARS-CoV-2, and the authors note similarities between this pattern and that previously reported in human patients treated with methotrexate chemotherapy.
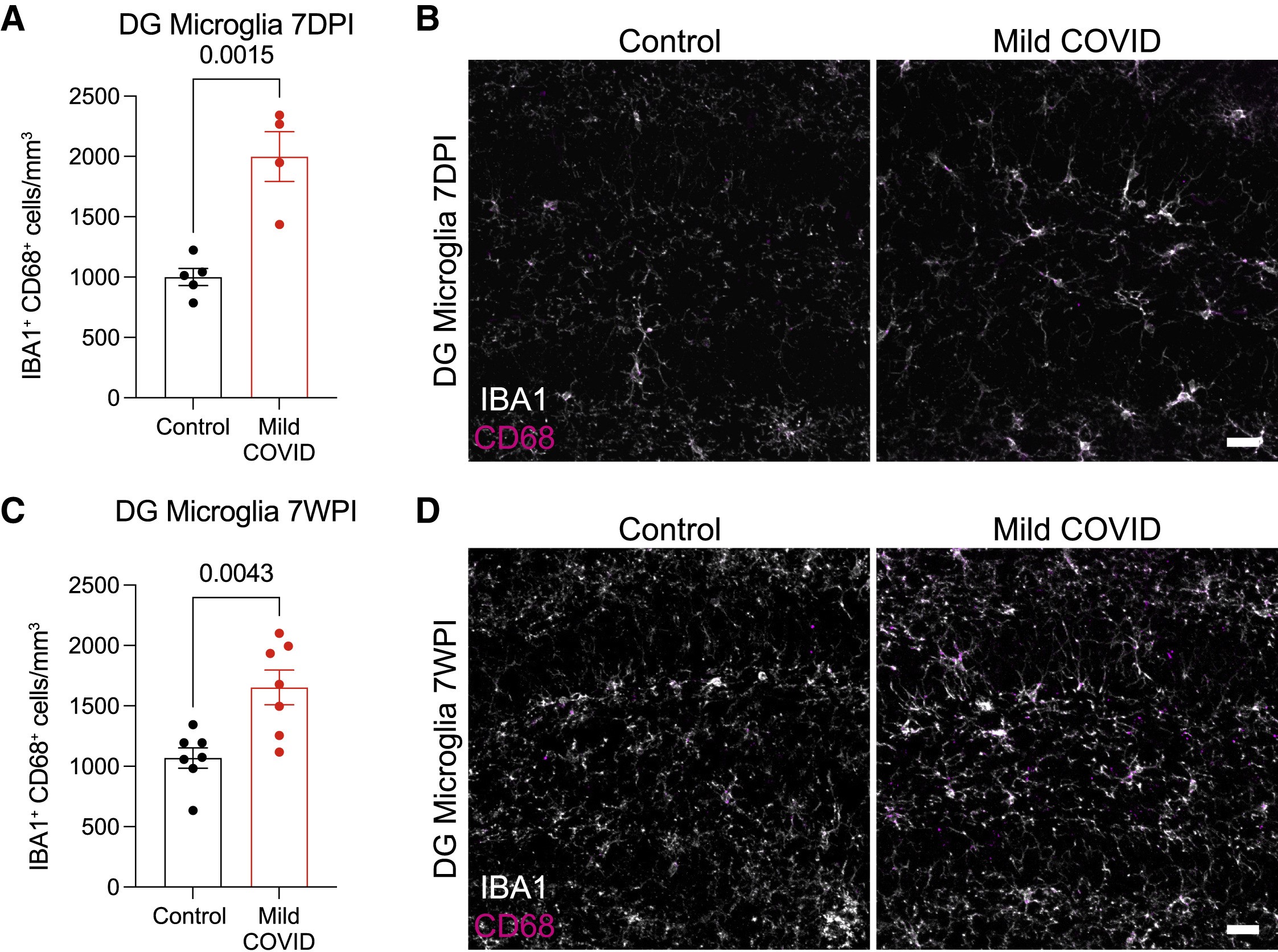
Figure 2: Reactive microglia quantification in the dentate gyrus (DG) of control and mild COVID mice 7 days post-infection (7DPI, top row) and 7 weeks post-infection (7WPI, bottom row). Scale bars = 50 μm. © 2022 Fernández-Castañeda et al., licensed under CC BY 4.0.
Implications and limitations
Despite the variability in viral strains, severity of symptoms, and a myriad other factors, our understanding of long COVID and its public health impact is growing. The persistence of cognitive symptoms long after the initial resolution of SARS-CoV-2 infection is understandably of great concern to both the public and clinical communities alike. In this paper, Fernández-Castañeda et al. have demonstrated similarities between COVID fog and chemo fog in an attempt to elucidate some of the neurological mechanisms that may underlie both syndromes. Increases in certain neuroinflammatory markers and microglial reactivity were found to persist following COVID, even in mild cases, and long after the initial infection was resolved. As a new and emergent condition, additional data is required, but these results may guide further examination of the long-term consequences of COVID, and ultimately provide therapeutic targets for those who continue to suffer long after the initial fever, sore throat, cough, and other symptoms have faded from prominence.
About the Author
About the Author

Andrew Davies has a BSc in Neuroscience and Biology from the University of Toronto and an MSc and PhD in Neuroscience from the University of Western Ontario. In addition to a number of first authored and co-authored publications, he has over 10 years of hands-on research experience in a number of fields, including electrophysiology, imaging, surgery, behavioral studies, and immunology.
References
- Nasserie T, Hittle M, Goodman SN. Assessment of the frequency and variety of persistent symptoms among patients with COVID-19: a systematic review. JAMA Netw Open. 2021;4(5):e2111417. DOI: 10.1001/jamanetworkopen.2021.11417.
- Davis HE, Assaf GS, McCorkell L, Wei H, Low RJ, Re’em Y, et al. Characterizing long COVID in an international cohort: 7 months of symptoms and their impact. EClinicalMedicine 2021;38:101019. DOI: 10.1016/j.eclinm.2021.101019.
- Fernández-Castañeda A, Lu P, Geraghty AC, Song E, Lee M-H, Wood J, et al. Mild respiratory COVID can cause multi-lineage neural cell and myelin dysregulation. Cell. 2022;185(14):2452-68. DOI: 10.1016/j.cell.2022.06.008.