Combination Therapy for Cancer Treatment: Recent Preclinical Advances
Blog post by Nina Culum, MSc
Despite decades of research and treatment advances, cancer remains a global health problem. Reduced access to care during the COVID-19 pandemic has led to delays in cancer diagnosis and treatment for many, which could lead to increased mortality in the coming years, further emphasizing the need to develop effective treatments [1]. The combination of anti-cancer drugs, or combination therapy, has been hailed as a “cornerstone of cancer therapy” since it targets key pathways synergistically, thereby enhancing efficacy compared to monotherapies [2]. Combination therapy could reduce drug resistance while providing anti-cancer benefits such as reducing tumor growth and metastatic potential [2, 3]. Furthermore, if at least one of the drugs already has regulatory approval, both research and therapy costs can be reduced [2].
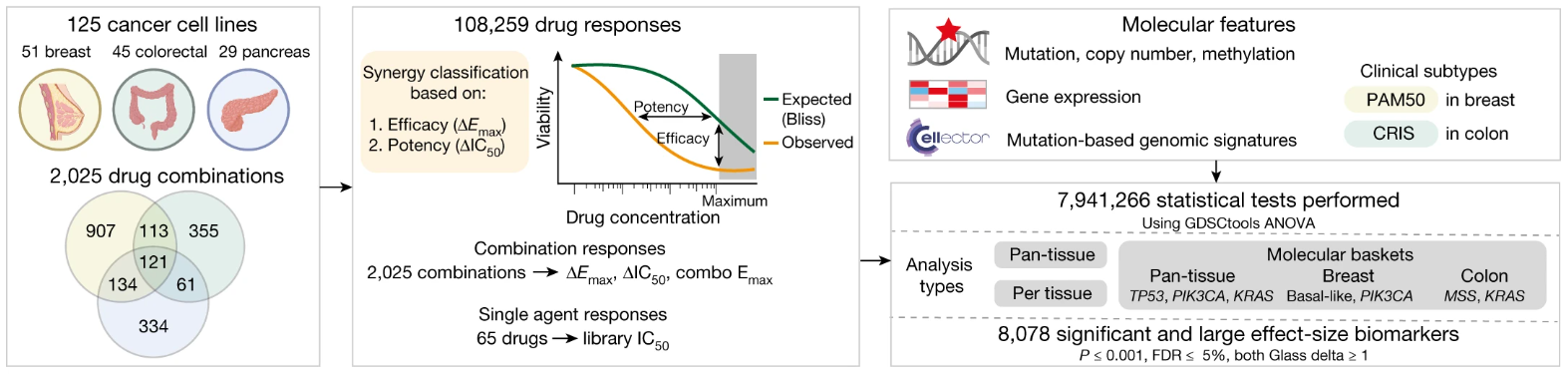
Unfortunately, identifying effective anti-cancer drug combinations is challenging since the number of possible combinations exceeds what can be tested clinically [3]. Jaaks et al. have systematically identified active drug combinations using the Genomics of Drug Sensitivity Cancer cell line screening platform to measure the effects of 2025 pairwise drug combinations in breast, colon, and pancreatic cancer cell lines, the results of which were published in Nature earlier this year [3]. This publication review will take a closer look at how these combination therapies were screened, as well as which therapies were determined to be effective.
Combination therapy screening
Approved chemotherapeutics and targeted agents, drugs in clinical development, and investigational compounds were selected for each tissue, and were screened with an “anchored” approach. The observed combination response of the cells was compared to the Bliss independence-predicted response based on monotherapy activity. Drug combinations were evaluated based on shifts beyond Bliss in potency (i.e., increased sensitivity) or efficacy (i.e., reduced cell viability). Interestingly, the authors found that although the type of tissue has some effect on combination response, it is not a major driver of variance in combination response by itself, and all combinations could either be classified as broadly, minimally, or variably active.
Schematic of drug combinations screened in breast, colon, and pancreas cancer cell lines. © 2022, Jaaks et al., licensed under CC BY 4.0.
Synergy is rare
Jaaks et al. determined that 5.2% of more than 100,000 combination-cell line pairs demonstrated synergy, with the highest rate in pancreas, then colon and breast. Furthermore, chemotherapeutic-chemotherapeutic combinations demonstrated the lowest synergy rates, while targeted-targeted combinations demonstrated the highest. Synergy was also significantly enriched when chemotherapeutics were paired with drugs that targeted apoptotic signaling and cell cycle inhibitors.
Irinotecan and rabusertib for colon cancer treatment
Campothectin, a TOP1 inhibitor, and AZD7762, a CHEK1/2 inhibitor, were identified as one of the top synergistic combinations. Therefore, as preclinical proof of concept, the authors validated the combination of irinotecan, a TOP1 inhibitor approved for colon cancer treatment, and rabusertib, a CHEK1-selective inhibitor with an acceptable safety profile (in phase I clinical trials) in mouse models of colon cancer. In these experiments, combination therapy demonstrated more pronounced anti-tumor effects compared to treatment with irinotecan alone, leading to apoptosis and suppressed tumor xenograft growth. The authors noted that there is also potential to follow up with other identified synergistic drug combinations.
Clinical implications
Importantly, the results from this study provide a foundation from which potent anti-cancer combination therapies can be discovered and developed. The data presented by Jaaks et al. also enrich existing cancer cell line datasets and could improve computational approaches that currently lack in training sets. The authors note that drug combinations should also be tested in non-cancer cell lines to estimate clinical toxicity.
About the Author
About the Author

Nina Culum graduated from the University of Western Ontario with a Master of Science in physical and analytical chemistry. During her graduate studies, she fabricated plasmonic nanohole arrays to capture extracellular vesicles and detect cancer by surface-enhanced Raman spectroscopy. Prior to attending UWO, Nina completed her Bachelor of Science in chemistry at the University of Waterloo.
References
- Siegel RL, Miller KD, Fuchs HE, Jemal A. Cancer statistics, 2022. CA Cancer J Clin. 2022;72(1):7-33. DOI: 10.3322/caac.21708.
- Mokhtari RB, Homayouni TS, Baluch N, Morgatskaya E, Kumar S, et al. Combination therapy in combating cancer. Oncotarget. 2017;8:38022-43. DOI: 10.18632/oncotarget.16723.
- Jaaks P, Coker EA, Vis DJ, Edwards O, Carpenter EF, et al. Effective drug combinations in breast, colon, and pancreatic cancer cells. Nature. 2022;603:166-73. DOI: 10.1038/s41586-022-04437-2.