Extracellular Vesicles as Breast Cancer Biomarkers: Diagnostic Potential and Recent Mechanistic Findings
Blog post by Nina Culum, MSc
Breast cancer is recognized as the most prevalent type of cancer in the world, and is the most commonly diagnosed cancer among American women [1, 2]. Triple-negative breast cancer (TNBC), which lacks estrogen, progesterone, and human epidermal growth factor receptor 2 (HER2), is particularly aggressive, challenging to treat, and accounts for 15-20% of all breast cancers [3]. In this blog, we review a study by Xie et al. published in Nature Communications, in which they examined the transforming growth factor-β (TGF-β) targetable signaling pathway and its role in malignant cancer progression and immune suppression [4].
Tumor-derived extracellular vesicles as non-invasive cancer biomarkers
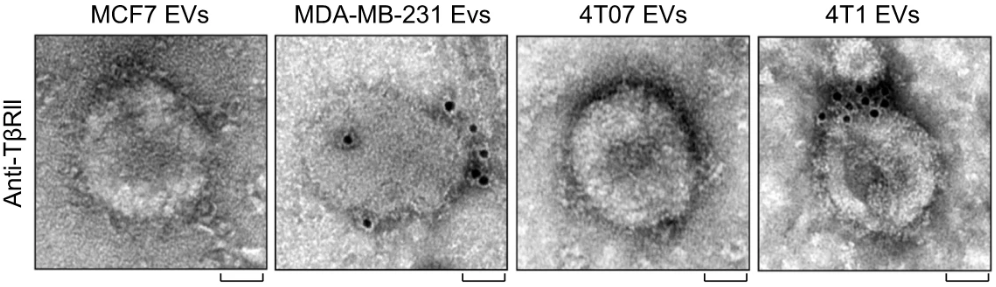
We discussed extracellular vesicles and their applications to vaccine development in a recent blog post, but they are also of interest as cancer biomarkers. Tumor-derived extracellular vesicles (TEVs) carry bioactive cargo such as nucleic acids and proteins from their parent cells during metastasis and mediate intercellular communication, making them potential diagnostic markers for tumor development [5]. Although exosomal TGF-β released from cancer cells has been shown to favor invasive growth and metastasis, Xie et al. noted that it is unknown whether TEVs containing TGF-β can influence T cell exhaustion in the tumor microenvironment [4]. The authors also demonstrated that high levels of TGF-β type II receptors (TβRII) mark circulating extracellular vesicles (crEVs) from malignant breast tumors (Figure 1).
Figure 1: Transmission electron microscopy images of breast cancer cell line-derived extracellular vesicles immunogold-labeled with TβRII antibodies (scale bars = 50 nm), in which many of the TβRII molecules were found to be anchored to vesicle membranes. © 2022, Xie et al., licensed under CC BY 4.0.
Diagnostic and prognostic relevance of TβRII+ crEVs in breast cancer
The secretion of TβRII by extracellular vesicles was investigated both in murine models of breast cancer as well as in breast cancer patients. In mice, blood levels of TβRII+ crEV positively correlated with tumor burden, increased proportionally with tumor size, and were associated with metastasis. In the sera of healthy human donors, crEVs exhibited very low baseline TβRII positivity, which was much higher in 89% of breast cancer patients. Additionally, the amount of TβRII in crEVs was the highest in TNBC patients compared to HER2+ and luminal patients. Patients with distant metastasis also exhibited higher amounts of TβRII in crEVs than those without metastasis.
The authors determined that the percentage of TβRII+ crEVs could be a classifier for breast cancer, as they were able to differentiate between breast cancer patients and healthy donors with 92% accuracy, 93% sensitivity, and 90% specificity. Furthermore, patients with lower levels of TβRII+ crEVs also exhibited improved overall survival and metastasis-free survival compared to those with higher TβRII+ crEVs levels. Results from these experiments indicate that TβRII is a noninvasive biomarker for metastatic breast cancer with both diagnostic and prognostic potential.
Effects of TβRII on metastasis, anti-tumor immunity, and T cell exhaustion
This paper also reports several mechanistic studies that examine the effects of TβRII+ crEVs on metastatic tumor outgrowth and anti-tumor immunity. The authors determined that TβRII transferred by TEVs induces TGF-β/SMAD activity, and that TβRII+ extracellular vesicles play a critical role in cancer stemness and metastasis. Furthermore, breast cancer cells pre-treated with TβRII+ extracellular vesicles were found to be more resistant to the chemotherapy drugs paclitaxel and doxorubicin. The authors also found an inverse correlation with the amount of TβRII+ crEVs and interferon-γ production, suggesting that TβRII+ secretion from tumor cells may negatively affect anti-tumor immunity.
Lastly, the authors investigated the relationship between TβRII and CD8+ T cell exhaustion. TβRII depletion in tumors was associated with higher frequencies of effector cytokines, higher levels of inhibitory receptors, and decreased proliferating capacity of CD8+ T cells, suggesting that TβRII from TEVs may induce T cell exhaustion and suppress anti-tumor immunity. Mechanistically, the authors determined that SMAD3 partners with T cell factor-1 (TCF1) to induce CD8+ T cell exhaustion (Figure 2).
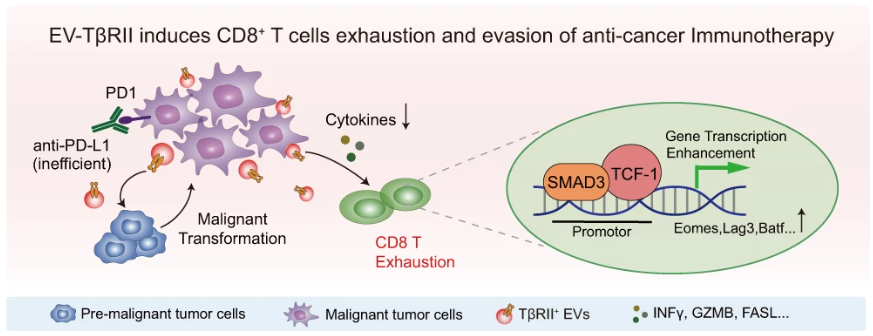
Figure 2: Proposed mechanism of TβRII-mediated CD8+ T cell exhaustion and anti-tumor immunity evasion, in which TβRII+ TEVs stimulate TGF-β/SMAD activation in recipient cells, are up-taken in adjacent low-grade tumor cells to initiate epithelial-to-mesenchymal transition and increase tumor stemness, drug resistance, and metastasis. TβRII is delivered to CD8+ T cells, inducing SMAD3 activation, which cooperates with TCF1 to impose T cell exhaustion and anti-tumor immunity dysregulation. © 2022, Xie et al., licensed under CC BY 4.0.
Novelty and outlook
Xie et al. have elucidated a mechanism by which breast cancer cells can regulate T cell exhaustion, and concluded that reducing TβRII cargo from extracellular vesicles could improve immunotherapy resistance, a mechanism that has been unrecognized to date. The mechanistic studies reported herein may also explain why metastatic breast cancer patients exhibit increased T cell exhaustion. Additionally, the authors have identified TβRII as a potential biomarker for the early detection of metastatic breast cancer, which should be explored further in the future.
About the Author
About the Author

Nina Culum graduated from the University of Western Ontario with a Master of Science in physical and analytical chemistry. During her graduate studies, she fabricated plasmonic nanohole arrays to capture extracellular vesicles and detect cancer by surface-enhanced Raman spectroscopy. Prior to attending UWO, Nina completed her Bachelor of Science in chemistry at the University of Waterloo.
References
- Breastcancer.org [Internet]. Breast cancer facts and statistics. 2022 Jul 14 [cited 2022 Aug 12]. Available from: https://www.breastcancer.org/facts-statistics.
- World Health Organization [Internet]. Breast cancer. 2021 Mar 26 [cited 2022 Aug 12]. Available from: https://www.who.int/news-room/fact-sheets/detail/breast-cancer.
- Almansour NM. Triple-negative breast cancer: a brief review about epidemiology, risk factors, signaling pathways, treatment and role of artificial intelligence. Front Mol Biosci. 2022;9:836417. DOI: 10.3389/fmolb.2022.836417.
- Xie F, Zhou X, Su P, Li H, Tu Y, et al. Breast cancer cell-derived extracellular vesicles promote CD8+ T cell exhaustion via TGF-β type II receptor signaling. Nat Commun. 2022;13:4461. DOI: 10.1038/s41467-022-31250-2.
- Qiao F, Pan P, Yan J, Sun J, Zong Y, et al. Role of tumor‑derived extracellular vesicles in cancer progression and their clinical applications (review). Int J Oncol. 2019;54(5):1525-3. DOI: 10.3892/ijo.2019.4745.