From Trauma to Binge Eating: How Early Life Experiences Impact the Leptin System
Blog post by Jasmin Skinner
The first six years of a child’s life are crucial for their development, and traumatic events during this period can have long-lasting effects on their learning abilities and overall health in adulthood (1). For example, previous clinical studies have noted that early life trauma (ELT) is a risk factor for the development of maladaptive or emotional eating practices in adulthood, such as binge eating. Binge eating disorder (BED) is characterized by recurring episodes of binge eating accompanied by feelings of a lack of control. In addition to being the most common type of eating disorder, adults with BED frequently report a history of ELT, such as family conflict, familial loss, or economic distress, highlighting the importance of early development on eating patterns (2).
While previous studies have examined how early traumatic experiences alter the feeding-related hormonal system within the hypothalamic-pituitary-adrenal (HPA) axis resulting in maladaptive eating behaviors, a comprehensive understanding of these neuronal pathways has yet to be established. One growth factor in particular, leptin, appears to be particularly relevant as a surge in circulating leptin levels during postnatal development regulates crucial gene expression and synaptic connections for developing hypothalamic circuits. As the hypothalamus regulates feeding behavior, it is therefore possible that early traumatic experiences may disrupt the leptin system and adversely affect the development of hypothalamic circuits that regulate feeding behavior. However, until recently, this leptin-dependent neuronal mechanism had not been properly characterized. In this blog, we review a recent study on the circuitry behind this ELT-induced maladaptive eating, and highlight new findings that may lead to the development of novel therapeutic strategies for binge eating disorders (2).
This webinar features Dr. Ileana Hanganu-Opatz, Dr. Jastyn A. Pöpplau, and Marilena Hnida, MSc, as they share their research on prefrontal networks in developing head-fixed mice, and the role of these networks in cognition and models of neurodevelopmental diseases. Watch this webinar HERE.
Early life trauma and binge-like eating protocol & study design
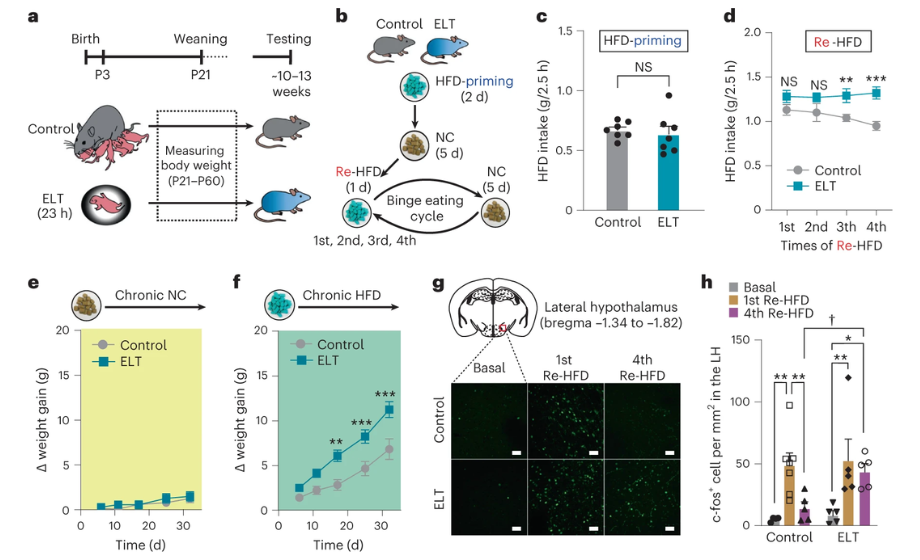
To determine the neurological changes associated with ELT-induced maladaptive eating, Shin et al. developed a strain of mice that allowed for the visualization of leptin receptor (Lepr)-positive neurons. To achieve this, they crossed a Lepr-Cre mouse strain with an Ai14 strain, then backcrossed these mice with wild-type C57BL/6J mice for multiple generations to refresh their genetic background (3). Once a suitably diverse strain had been confirmed, pregnant mice were placed in individual housing after 14-16 days of pregnancy (2).
To induce early life trauma, pups were separated from their mothers and littermates at postnatal days 3-4 for approximately 23 hours. They were then returned to the litter and weaned with control pups after postnatal day 21; control pups were never separated from their litter (2).
Prior to the start of this binge-like eating paradigm, mice were fed a standard diet of 18% fat (NC). Once these mice were at a suitable age (10-13 weeks), all were placed into individual housing and offered free access (ad libitum) to the high fat diet (HFD, 60% fat) for two days, and then switched to the standard diet for five days. On day 8, the mice were re-exposed to this HFD again, now with food intake being monitored. After 24 hours, the HFD was again removed and replaced with the standard diet, thus completing the first binge eating cycle. Subsequent cycles used a six day NC-only diet, which was followed by the HFD for 24 hours. Food intake as well as body weight were measured throughout, and O2 consumption, CO2 production, and respiratory exchange rate were measured after HFD reintroduction to determine energy expenditure (2).
After the binge eating paradigm was concluded, mice underwent a locomotion assessment, as well as an open field test and novel object recognition test to determine whether ELT had any long-lasting impact on stress responses. Glucose tolerance and blood pressure were monitored throughout the study, and, following the completion of the test protocol, immunohistochemistry, RNA in situ hybridization, and circuit mapping quantification were performed following euthanization (2).
Early life trauma induces binge-like eating in adult mice
Immediately following separation, ELT pups had lower leptin and higher corticosterone levels when compared with controls. As leptin and corticosterone are implicated in long-term regulation of energy balance and stress-induced HPA axis activity, respectively, this suggested that the early-life trauma paradigm was successful (3,4). Despite this, once the mice reached adulthood, no differences in anxiety-like behaviors [increased vigilance and heart rate, freezing/hypoactivity, and suppressed food consumption (5)], locomotion, glucose metabolism, and baseline leptin/corticosterone levels were observed between the two test groups (2).
Comparing feeding behaviors, the ELT group had a greater tendency towards binge-like eating. When the HFD was repeatedly reintroduced (re-HFD), the ELT mice showed binge eating tendencies that were particularly aggravated in the fourth cycle of re-HFD, but not in the first. These mice also displayed rapid weight gain when fed the high fat diet, but not when offered continual access to the normal food supply, suggesting that early life trauma primes these mice for the development of high fat diet-induced obesity, primarily from overconsumption (2).
How do leptin receptors within the lateral hypothalamus affect binge-like eating and obesity?
While it’s clear that leptin plays a significant role in binge-like eating behavior observed after early life trauma, the role of its receptor is not as well understood. While activation of leptin receptors (Lepr) results in satiety (6), the downregulation of Lepr within the lateral hypothalamus appears to mediate binge-like eating and/or obesity, but the mechanisms behind this phenomenon are not yet fully characterized (2).
The authors first sought to determine where Lepr-positive neurons were located using dual-fluorescence in situ hybridization (FISH). The results demonstrated that these neurons were located within the caudal lateral hypothalamus, and were predominantly (80.7%) GABAergic (7). Once the location of these neurons was confirmed, the authors then sought to determine whether re-HFD induced preferential expression of c-fos, a gene involved in cell proliferation and differentiation in response to extracellular stimuli, within the lateral hypothalamus leptin receptor (LHLepr) neurons (8). Following gene expression analysis, reintroduction to a high-fat diet enhanced c-fos expression in the LHLepr neurons by 42%, indicating that these neurons are preferentially activated under binge-like eating conditions. Additionally, in a shRNA-mediated knockdown of Lepr, c-fos expression increased after the first and fourth introduction to the HFD, whereas the control mice did not exhibit increased c-fos induction after the fourth cycle. To further characterize whether a reduction in Lepr signaling truly does result in increased LHLepr neuronal activity, whole-cell patch-clamp recordings of these neurons were obtained following injection of a Lepr shRNA adeno-associated virus. As both the ELT mice and the control mice expressing Lepr shRNA demonstrated increased LHLepr activity, there is strong evidence that early life trauma is concurrently associated with reduced Lepr signaling, which in turn results in increased LHLepr neural activity upon repeated exposure to a high fat diet. Since reduced Lepr signaling results in increased lateral hypothalamic neuronal activity, it is likely that this receptor plays an inhibitory role on these neurons and their downstream targets, but the question of what neurons are being targeted still remains (2).
To determine the functional target of these LHLepr neurons, a series of tracer studies were then performed. It was determined that these neurons primarily form synapses with three downstream targets: the medial preoptic area (MPA), ventral tegmental area (VTA), and the ventrolateral periaqueductal gray (vlPAG). However, upon selective inhibition of LHLepr–vlPAG neurons and LHLepr–MPA neurons, the former appeared to play a more important role in binge-like eating regulation, indicating that vIPAG is the primary functional downstream target in this pathway. This was further supported by additional comparison of the downstream effects of silencing vlPAG versus VTA, during which it was observed that the LHLepr –vlPAG circuit specifically encodes high-fat diet consumption, but did not impact food intake during the priming stage, or when ample NC food was available following mild food deprivation. Additionally, inhibition of the LHLepr–vlPAG circuitry reverses and prevents the augmentation of binge-like eating habits associated with early life trauma, providing clear evidence to support this hypothesis (2).
Figure 1: Brief overview of study protocol (a,b). High fat diet intake of early life trauma (ELT) mice increased significantly after the fourth reintroduction to the HFD (c,d), as did total weight gain (e,f). C-fos expression was also increased in the ELT mice (g,h), most significantly after the first re-introduction to the HFD. © 2023 Shin et al., licensed under CC BY 4.0.
While it is clear that the vlPAG is the target of the LHLepr neurons, the molecular identity of the neurons that receive these signals has not yet been described. This region of the brain largely expresses the endogenous opioid peptide Penk, which has been observed to regulate pain sensation, food intake, and reward processing. Through a combination of virus-mediated input tracing and FISH, it was revealed that the predominant inputs from the lateral hypothalamus to vlPAGPenk are from the LHLepr neurons, suggesting that the vlPAGPenk is the most likely downstream target for the induction of binge-like eating resulting in obesity (2).
Study limitations and future directions
While the delineation of this neuronal pathway could provide future targets for treatment of binge eating disorder, additional research is required to validate and extend these findings. As mice were housed independently and tests were performed during their natural sleeping cycle, results could potentially be skewed as both these conditions can impact the behavioral and stress responses observed (9). Ultimately, this research demonstrates that early life trauma can substantially impact the metabolic pathways and behaviors observed in adult mice, but whether this effect will be translatable to humans has yet to be determined.
About the Author
About the Author

Jasmin Skinner is an undergraduate student at the University of Western Ontario completing a Specialization in Biology and a Minor in Chemistry, with focused interest in applying these concepts to environmental conservation. As a lover of the outdoors and the arts, much of her time is spent in nature and within the local London art community, creating and connecting with all walks of life. After graduating, she hopes to continue her passion of finding unconventional solutions to environmental issues by working with nature, not against it.
References
- CDC (Centers for Disease Control and Prevention) [Internet]. Atlanta, GA: CDC; c1946. Early brain development and health; 2023 Feb [cited 2023 March]. Available from: https://www.cdc.gov/ncbddd/childdevelopment/early-brain-development.html
- Shin S, You IJ, Jeong M, Bae Y, Wang XY, Cawley ML, et al. Early adversity promotes binge-like eating habits by remodeling a leptin-responsive lateral hypothalamus-brainstem pathway. Nat Neurosci [Internet]. 2022 December [cited 2023 March]; 26:79-91. Available from: https://www.nature.com/articles/s41593-022-01208-0#citeas. doi: 10.1038/s41593-022-01208-0
- Kelmenson P. How to refresh your mutant or transgenic mouse strains [Internet]. Bar Harbor, Maine: The Jackson Laboratory. 2018 April – [cited 2023 March]. Available from: https://www.jax.org/news-and-insights/jax-blog/2018/april/how-to-refresh-your-mutant-or-transgenic-mouse-strains#:~:text=The%20way%20to%20mitigate%20the,to%20the%20inbred%20control%20strain.
- Mishima Y, Shinoda Y, Sadakata T, Kojima M, Wakana S, Furuichi T. Lack of stress responses to long-term effects of corticosterone in Caps2 knockout mice. Sci Rep [Internet]. 2015 March [cited 2023 March]; 5:8932. Available from: https://www.nature.com/articles/srep08932#:~:text=Corticosterone%20(CORT)%2C%20the%20major,in%20coping%20with%20stress9. doi: 10.1038/srep08932
- Lezak KR, Missing G, Carlezon WA. Behavioral methods to study anxiety in rodents. Dialogues Clin Neurosci [Internet]. 2017 June [cited 2023 March]; 19(2): 181-91. Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5573562/. doi: 10.31887/DCNS.2017.19.2/wcarlezon
- Klok MD, Jakobsdottir S, Drent ML. The role of leptin and ghrelin in the regulation of food intake and body weight in humans: a review. Obes Rev [Internet]. 2007 Jan [cited 2023 March]; 8(1):21-34. Available from: https://pubmed.ncbi.nlm.nih.gov/17212793/#:~:text=Leptin%20is%20a%20mediator%20of,a%20role%20in%20meal%20initiation. doi: 10.1111/j.1467-789X.2006.00270.x.
- Kelesidis T, Kelesidis I, Chou S, Mantzoros CS. Narrative review: the role of leptin in human physiology: emerging clinical applications. Ann Intern Med [Internet]. 2010 Jan [cited 2023 March]; 152(2): 93-100. Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2829242/#:~:text=Leptin%20exerts%20immediate%20effects%20by,neuropeptides%20to%20control%20food%20intake. doi: 10.1059/0003-4819-152-2-201001190-00008
- Rudolph U. GABAergic system. In: Offermanns S, Rosenthal W, editors. Encyclopedia of Molecular Pharmacology [Internet]. Berlin, Heidelberg: Springer; 2008 [cited 2023 March]. Pages 515-9. Available from: https://link.springer.com/referenceworkentry/10.1007/978-3-540-38918-7_61.
- Velazquez FN, Caputto BL, Boussin FD. c-Fos importance for brain development. Aging [Internet]. 2015 Dec [cited 2023 March]; 7(12): 1028-9. Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4712328/#:~:text=c%2
c-Fos importance for brain development – PMC. doi: 10.18632/aging.100862 - Arndt SS, Laarakker MC, van Lith HA, van der Staay FJ, Gieling E, Salomons AR, et al.
Individual housing of mice https://pubmed.ncbi.nlm.nih.gov/19303031/