Industry Insights with Crown Bioscience: Analyzing the Suppressive TME with In Vitro Based Assays
This episode of Industry Insights features Nataliia Beztsinna, PhD, and Marten Hornsveld, PhD, from Crown Bioscience, who recently presented a webinar on how the tumor microenvironment (TME) can impact clinical responses, as well as how patient-derived ex vivo tissue models can be used to maintain the native TME for preclinical testing. In this interview, they discuss trends in immuno-oncology research and describe how reconstituted TME and patient-derived ex vivo tissue assays can best be used within the drug development pipeline.
This interview has been edited slightly for clarity and conciseness.
In the webinar, we asked the audience what approaches are most suitable for their research, what kinds of immune cells are most relevant to their work, and which tumor indications are most relevant for their research in the context of the tumor micro-environment assay. What can you tell us about the polling results?
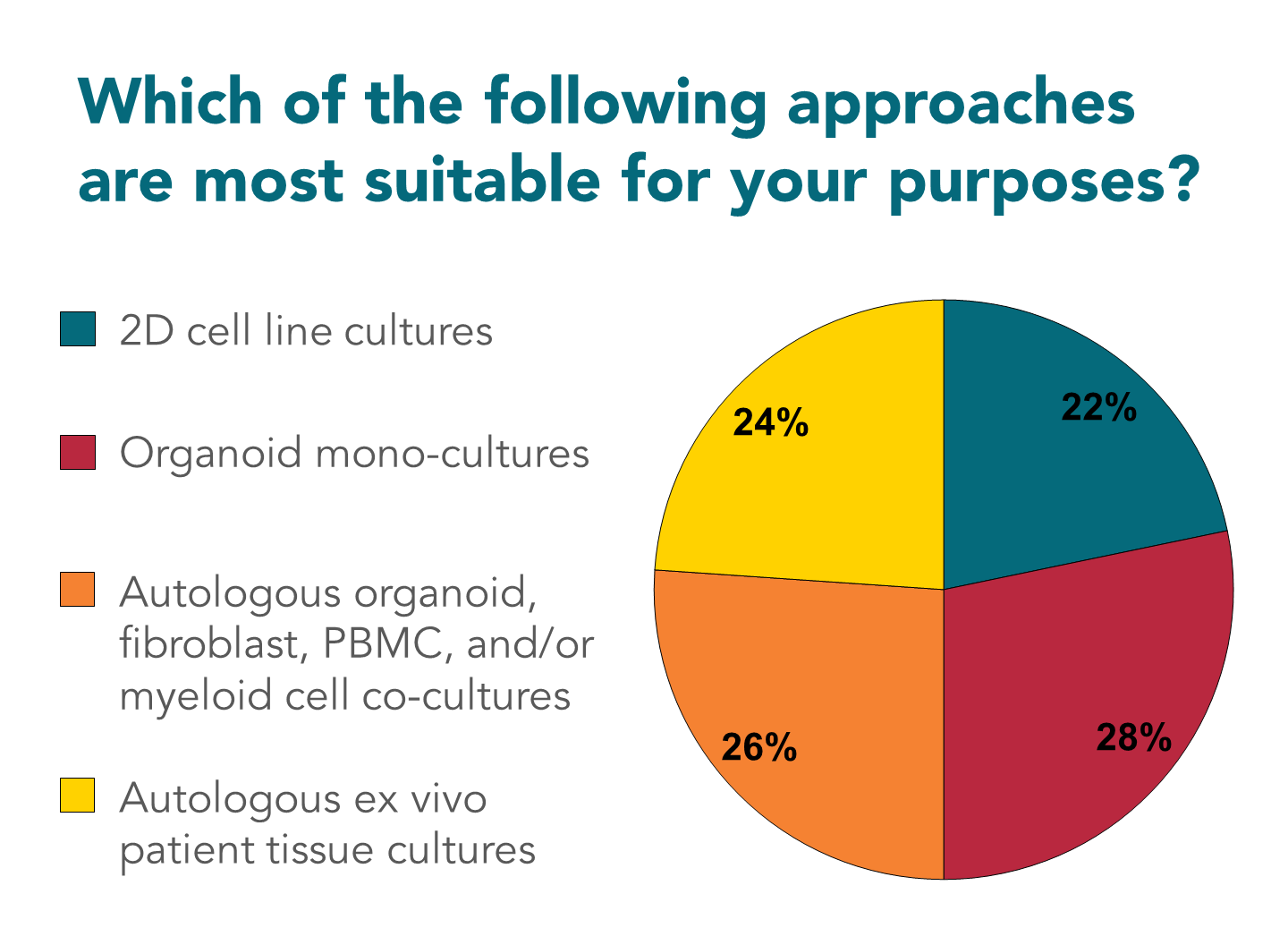
Figure 1: Polling results from the first audience poll in the webinar: “Analyzing the Suppressive TME in In Vitro Based Assays”.
Marten: I was a bit surprised that almost 50% of the people were still voting for the 2D cell culture or organoid monocultures. While we have been trying to convince people through the course of these lectures to go for more complex setups, there are many good reasons to go for 2D and 3D monocultures still, but I do think it was an interesting outcome. If you look at the interest in immuno-oncology, it’s almost always more than just one culture.
Nataliia: I was also surprised to see that 25% of people are still using 2D assays, but it takes time for the field to develop and 3D assays are a relatively new field that’s still developing. Complex 3D assays especially, not only organoids, but the assays that have multiple cell types included, like what Marten and I work with, it takes time for the field to accept them. It was also interesting to see what cell types people were thinking of using. I think the majority are still most interested in using T cells, but there were some other interesting cells used. What do you think, Marten?
Marten: For sure. In the end we forgot to include fibroblasts as an option, which was of course why everybody was tuning in for the cancer-immunity cycle talks. I think it’s nice to see that there’s a broad interest in different myeloid populations; it’s quite a complicated field that’s up and coming in immunotherapy approaches. T cells, of course, are already one of the decade-long favorites to study. So no surprise there that most people are interested in working with those.
There’s not as much interest in dendritic cells. Although there have been quite some promising studies recently where we can condition dendritic cells to start presenting tumor neoantigens, and then we transplant those. I think this is quite an interesting recent development in that field.
It’s also interesting to see that M1 and M2 macrophages apparently attract an equal amount of interest. Although I think for many people it will be hard to determine what polarization they want to target. This is also one of the questions in the podcast: how do you deal with those macrophages and, from this poll, it’s clear that this is something people are very eager to get into.
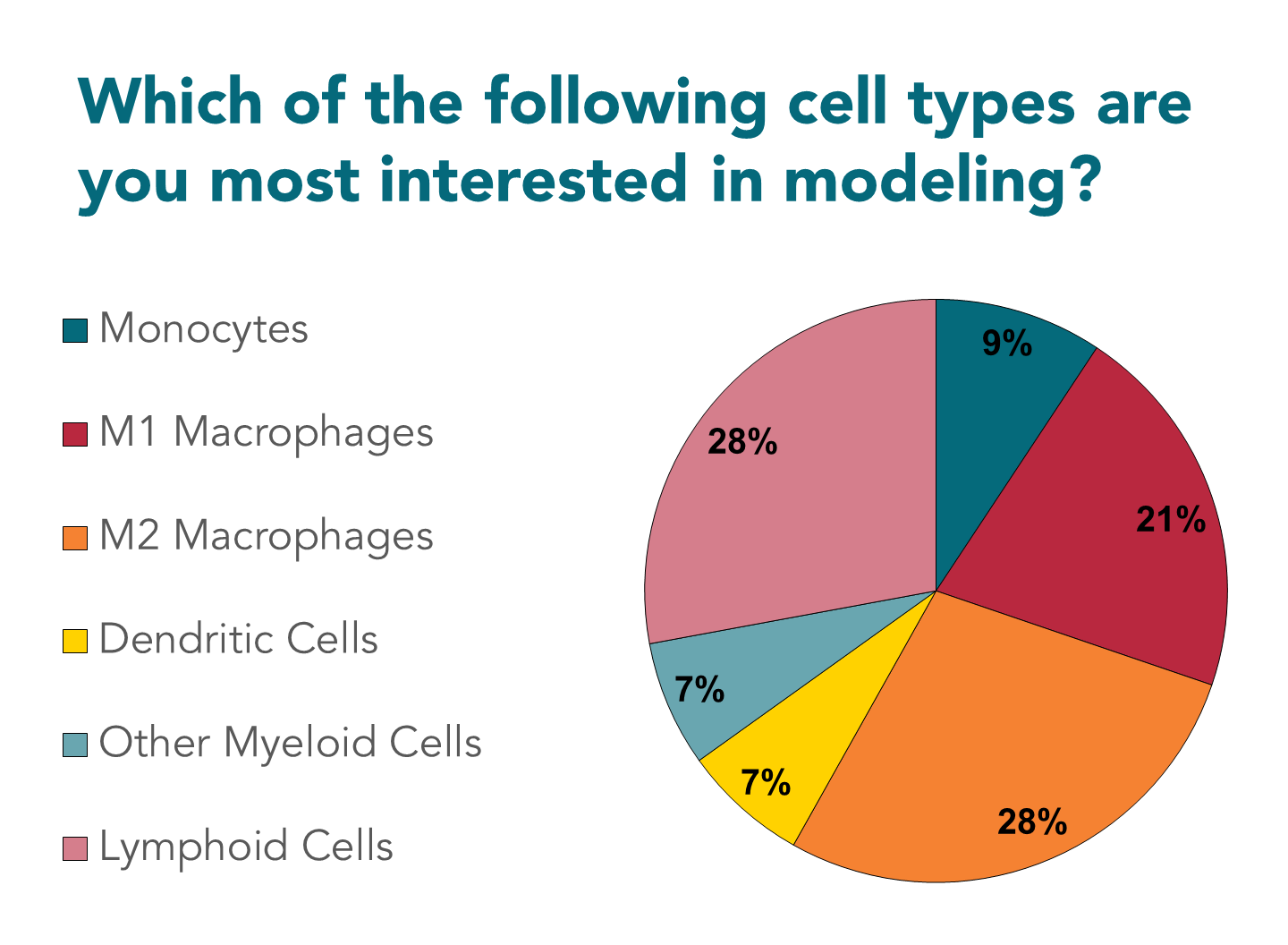
Figure 2: Polling results from the second audience poll in the webinar: “Analyzing the Suppressive TME in In Vitro Based Assays”.
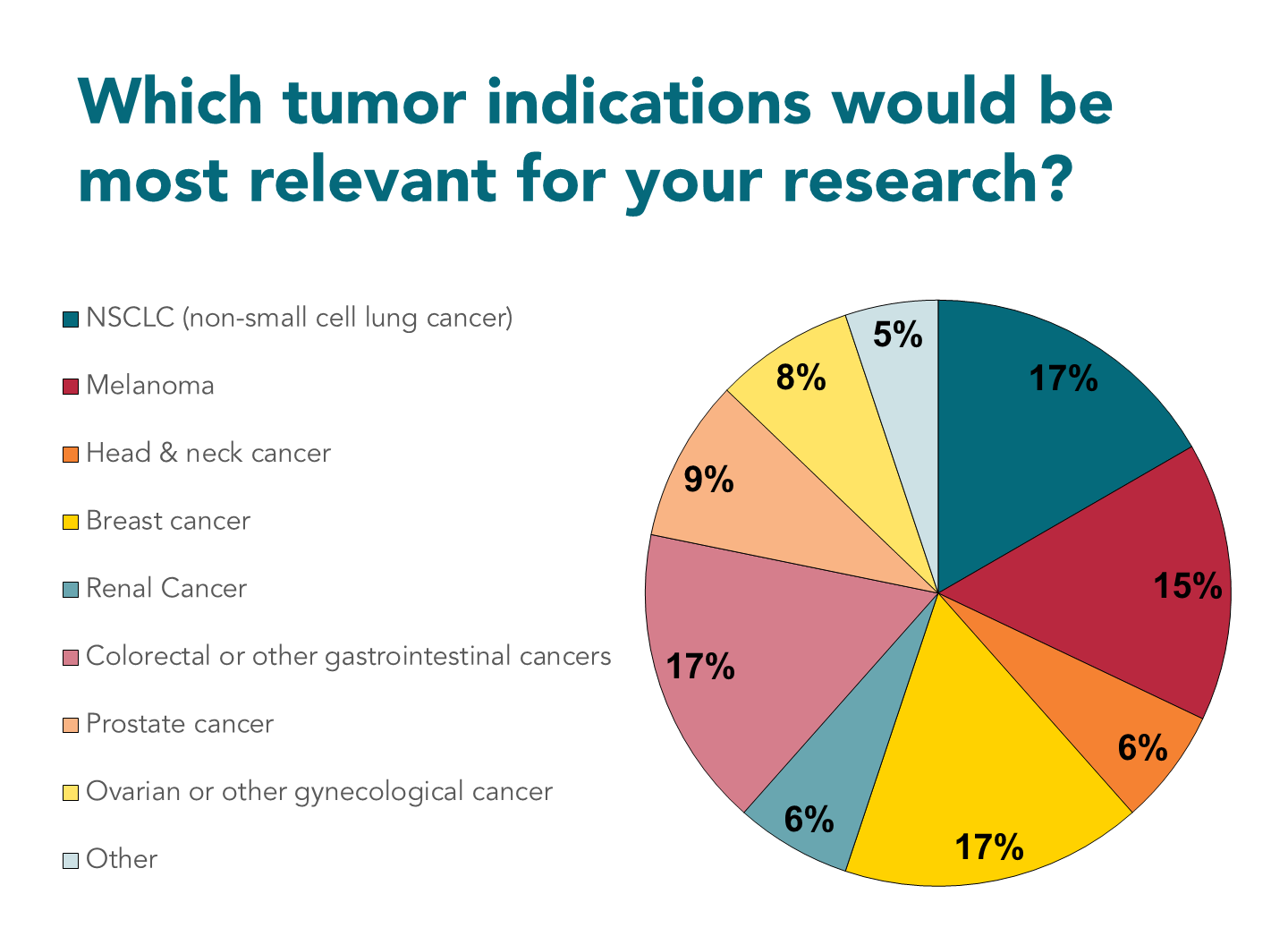
Figure 3: Polling results from the third audience poll in the webinar: “Analyzing the Suppressive TME in In Vitro Based Assays”.
Nataliia: Yeah. For me it was also interesting to see which tumor indications people are working with or what they’re interested in working with, because that’s more relevant for the native TME samples that we do receive in our team. The usual suspects, which are typically the non-small cell lung cancer, breast cancer, and melanoma, were the ones that scored highest in the poll.
On the other hand, gastric cancers and CRC were also quite well demanded in the assays as well as other, more rare types of cancers. Unfortunately we couldn’t include more options in these polls, but there are many more that we can do with these types of assays.
For what types of therapies do you think these assays are most useful?
Marten: Well, I mean, there’s a really broad range of applications that you can think of here. Also, to step back a little bit and comment on the 2D cell cultures and organoid monocultures because, of course, these are optimal models to study biochemistry.
“You know, if a protein binds to another protein, it doesn’t really matter what kind of 3D dimensions you grow your cell in. If those proteins are there, they will bind.”
So I think this is also the reason why many people still enjoy working with 2D cell cultures. They’re super practical, very good for discovery, screening, and cost-efficient. These kinds of developmental steps fit very well within the 2D cell line space.
But also, there is more and more evidence that you can combine discovery and mode of action tests in the same screens, using more complicated models like organoid models. Especially for co-cultures like the ones we’ve been presenting in our seminars, the co-cultures between myeloid cells, T cells, and fibroblasts are most suitable for mode of action studies. So not necessarily best in the drug discovery steps of the development pipeline. But if you already have some promising leads based on screening in basic models, these models become more relevant to study because the localization of the target that you want to engage with is heavily dependent on the 3D space and the culture conditions.
Also, especially in upcoming fields, like the antibody-conjugate fields or other antibody-based approaches, they really require you to be able to see where your antibody is going; is it binding to the right marker of interest, are they delivering into the set tissues, and how far and how deep these drugs penetrate into a tissue. This is really where the power of 3D cultures and the co-cultures comes in.
Speaking from my own expertise with fibroblasts, fibroblasts form physical barriers that you need to be able to bridge with your drug; they can metabolize drugs, they can affect cells and their survival responses. So in these cases, this Lego-like approach is very useful because you can get your cancer of interest, you mix them with the fibroblasts – most of the properties are there that you want to study. Then you can really get into the biology a bit more.
When we increase the complexity in the immune system, this becomes a little bit more tricky. In seminar 2, Marrit Putker demonstrated that you can make mixed organoid immune cell pairs, you know, autologous systems. The systems that I presented in my talk are all allogeneic, so you will run into limitations with T cell engagement with cells. We can do very basic killing assays, infiltration assays, these kinds of assays, they are still very suitable. But if you really want to go into this immune checkpoint inhibitor field, you need everything to actually be able to study this. This is really where Nataliia’s platform – the ex vivo patient tissue platform – comes in. I think I would be out of my league to start mansplaining this, so go ahead Nataliia.
Nataliia: Thanks, Marten. So if we are speaking about recapitulating the cancer-immunity cycle in a dish – in vitro – then in this case, the ex vivo assay would be our best bet, I would say, because it really gives a thumbprint of the patient tumor with all cell types that are represented there, including T cells, myeloid cells, fibroblasts and, of course, tumor cells. It’s all a complex interplay that is necessary there to observe drug effects from some very complex drugs, like immune checkpoint inhibitors. It is extremely difficult to see the effects in allogeneic setups from these molecules, like what Marten mentioned.
But in our ex vivo system we do see effects from these drugs, and we can measure them by some cytokine increase, so observing an indirect effect. But we also see a direct killing effect of the tumor, and that’s what makes this setup very valuable in the drug development process. When you do already have a drug candidate and you need to test it in real field-like conditions on a patient tissue, then this assay comes into play. We can test it on several different indications to see if there is a response to these types of molecules, like immune checkpoint inhibitors. It will depend, of course, a bit on the patient and on the indication.
“What we do see is that about 20% of the tissues that we receive, respond to at least the known immune checkpoint inhibitors. That also corresponds to what I’ve seen in the clinic, showing the translatability of this assay.”
One of the downsides here is that we have to test enough patient tissues to see those 20% responders. It’s not like we can take one patient sample and hope that the drug will work on it. It means that we need to test five, maybe sometimes 10 different patient samples to be able to see the drug’s effects.
What stages of drug development do you think are most relevant for the assays?
Nataliia: Marten already mentioned this, but more simplistic assays that do not involve a lot of cellular players can be used in the earlier stages. Maybe not for screening, but when the mechanism of action needs to be known a bit better in vitro. Regarding the ex vivo assays, it’s really almost at the door of clinical trials, where we can test the one or two of the best candidate compounds in a patient material. So think about it more like an in vitro clinical trial setup.
Marten: Yeah, I can really adhere to this, I think the reconstituted TME approaches are really the crossroads of academic discovery and the actual mode of action studies. Especially with the high-content imaging platforms, we can study how your drug affects a tumor in a lot of detail. This is really also dependent on the drug types and on the type of screen you want to perform because I think both the ex vivo platform and reconstituted TME, they’re both quite time consuming and labor intensive. This makes these procedures a little bit more expensive to perform, so it’s always better to already know a little bit about what you want to look for because unbiased screening for these kinds of setups is not very feasible or cost effective. You can put it somewhere in the direct development pipeline two or three years after you have your lead compounds and then are really eager to move on.