Novel Targets for Alzheimer’s Disease Treatment: Medin and PLD3
Blog post by Nina Culum, MSc
Alzheimer’s disease, the most common type of dementia worldwide, is becoming an increasingly serious global health problem [1, 2]. Owing largely to aging populations and increased life expectancy, the number of individuals living with dementia has more than doubled over the past three decades, imposing significant economic and disease burdens on both families and societies [2]. Despite this increased prevalence, a cure for Alzheimer’s disease remains elusive.
Extraneuronal amyloid plaques and intraneuronal neurofibrillary tangles composed of amyloid-β (Aβ) and hyperphosphorylated tau proteins, respectively, are thought to underlie neuronal damage in Alzheimer’s disease [3]. However, recent neuropathological studies have indicated that the disease is more heterogeneous than originally presumed. For example, α-synuclein and microvascular disease have now been implicated in the neuropathology of late-onset Alzheimer’s disease [3]. Therefore, progress in effective drug development is more likely to occur if more than one pathway is considered [4]. Two recent Nature publications have potentially identified new therapeutic targets for Alzheimer’s disease, including medin [5] and phospholipase D3 (PLD3) [6], which we summarize in this mini-review.
In this webinar, Dennis Turner, MA, MD, delves into dementia syndrome, the metabolic changes that occur, and the importance of proper physiological monitoring of animal models. WATCH NOW
The role of medin in Alzheimer’s disease
Study motivation: Medin, the most common human amyloid known to date, has been found to form vascular aggregates in aging wildtype mice, causing cerebrovascular dysfunction. Aggregates of medin have also been found postmortem in cerebral arterioles of patients with vascular dementia. Therefore, Wagner et al. sought to study the role of medin in mouse models of cerebral β-amyloidosis and postmortem human brain tissue to determine whether its increased levels are a cause or consequence of Alzheimer’s disease [5].
Key findings: In two transgenic Aβ precursor protein (APP) mouse models, staining with the MFG-E8 antibody, which has been shown to recognize extracellular medin aggregates in the vasculature of aging wildtype mice, revealed that medin extensively co-localizes with amyloid plaques (Figure 1). Additionally, medin deficiency was found to reduce cerebral Aβ angiopathy (CAA) as well as vascular Aβ deposition. Although the authors could not biochemically confirm the presence of medin in the mouse samples, these data demonstrate that the medin precursor protein MFG-E8 is highly enriched in the vasculature and increases further with CAA.
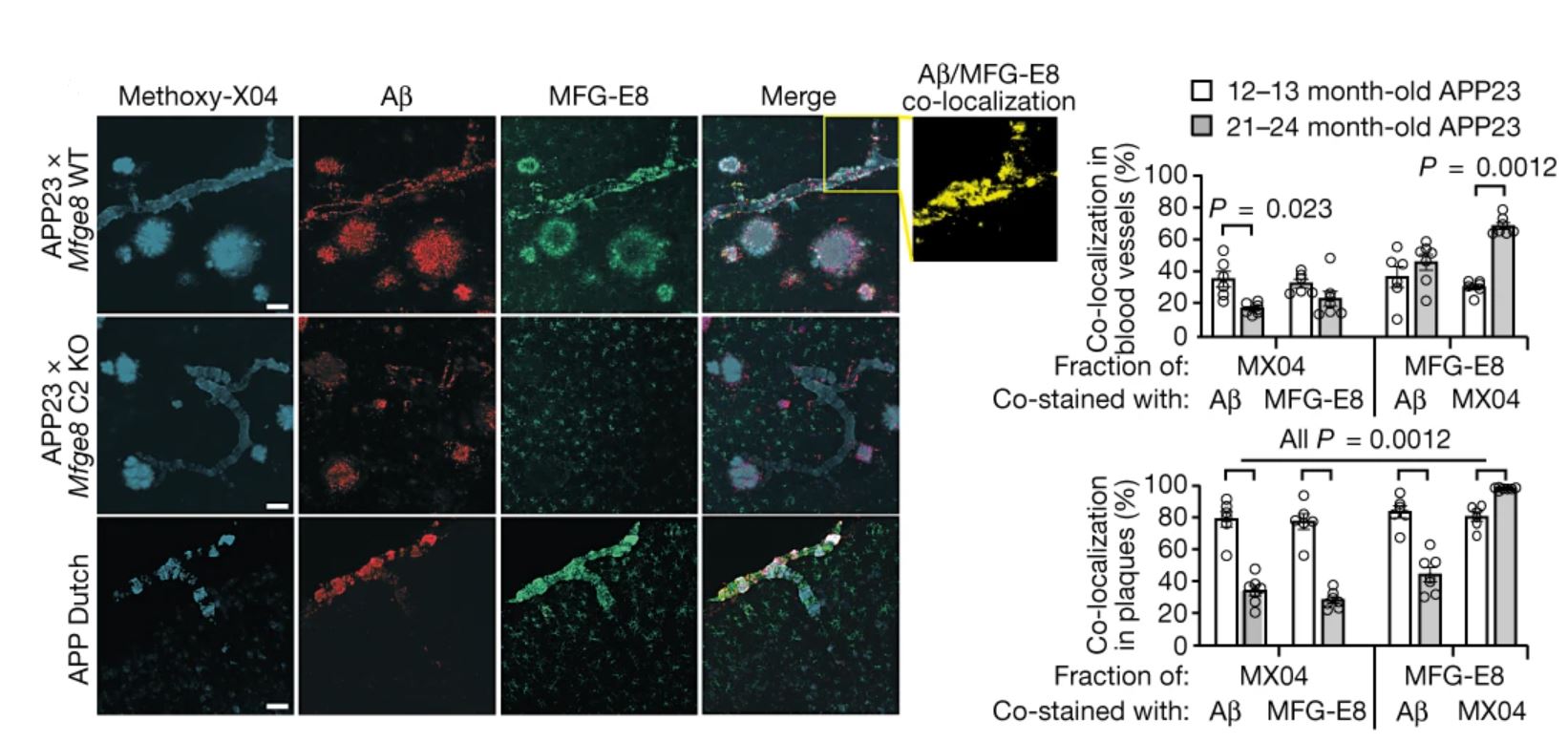
Figure 1: Cortical brain sections of APP mice stained for MFG-E8 and Aβ, as well as with the amyloid dye Methoxy-X04 (left) and colocalization quantification (right). Scale bars = 50 μm. © 2022 Wagner et al., licensed under CC BY 4.0.
MFG-E8 was also found to be highly enriched in the vasculature of postmortem brain tissue from patients with Alzheimer’s disease (Figure 2). Specifically, increased MFG-E8 and medin levels were associated with CAA in these samples. The authors also examined Mfge8 gene expression in patients enrolled in the Religious Orders Study/Memory and Aging Project (ROSMAP), and found that it was significantly increased in patients with Alzheimer’s disease compared to controls without dementia, though it did not vary in an age-dependent manner. Therefore, increased Mfge8 expression is likely to be a result of Alzheimer’s disease-associated pathology and not simply aging.
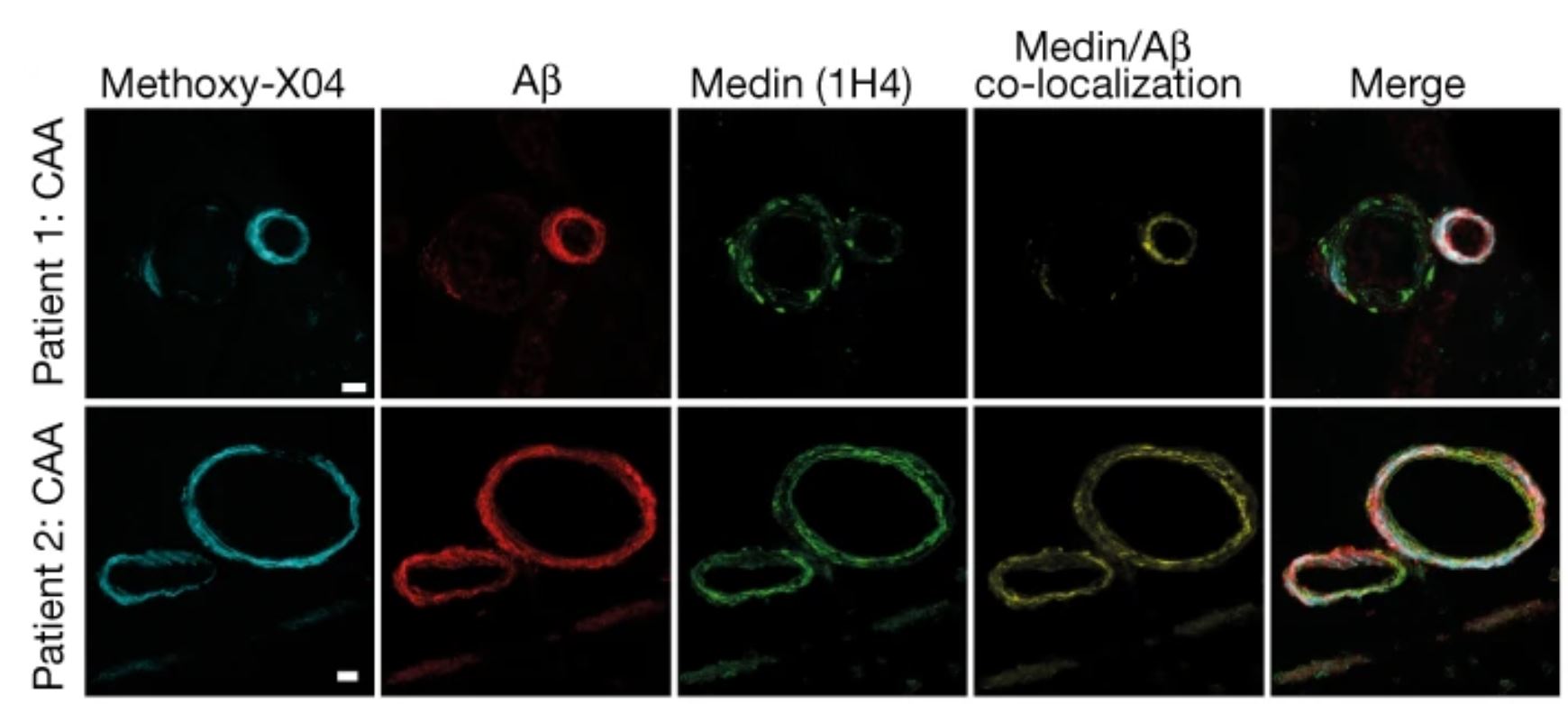
Figure 2: Human brain sections from patients with Alzheimer’s disease stained for medin and Aβ, as well as with Methoxy-X04. Scale bars = 20 μm. © 2022 Wagner et al., licensed under CC BY 4.0.
Additionally, elevated Mfge8 expression was significantly associated with increased cognitive decline in the ROSMAP cohort and most likely driven by vascular alterations in patients with Alzheimer’s disease, which was in line with the findings from the postmortem human brain samples. Lastly, the authors demonstrated mechanistically that Aβ and medin can interact directly and promote amyloid aggregation. Furthermore, in vivo experiments utilizing both mouse and human tissue extracts confirmed that medin aggregates can accelerate Aβ pathology through a heterologous seeding mechanism.
Conclusions and implications: It is becoming increasingly clear that Alzheimer’s disease pathogenesis has an important vascular component. In particular, medin may contribute to cognitive decline by altering vascular function and Aβ deposition. Therefore, targeting medin may represent a novel therapeutic approach to preserving brain function in patients with Alzheimer’s disease by improving vascular health.
In this webinar, Ashley Walker, PhD, discusses the vascular contributions to dementia and the role of age-related arterial stiffness on Alzheimer’s development. WATCH NOW
The role of axonal spheroids and PLD3 in Alzheimer’s disease
Study motivation: The development of effective Alzheimer’s disease therapies is largely focused on strategies for removing extracellular amyloid, but is hindered by a limited understanding of the links between Aβ deposition and neural network destruction. An important but understudied pathological hallmark of Alzheimer’s disease is enlarged neuronal processes found around Aβ deposition, traditionally known as dystrophic neurites or, more recently, plaque-associated axonal spheroids (PAASs). Thus, Yuan et al. aimed to assess their physiological importance in Alzheimer’s disease through high-resolution structural imaging, time-lapse intraviral calcium and voltage imaging, and computational modeling [6].
Key findings: In a mouse model of Alzheimer’s disease (i.e., 5xFAD mice), PAASs were found to be stable across month-long intervals, but although most increased in size over time, a substantial amount decreased in volume or disappeared without the loss of the parent axon. Subsequent in silico modeling and live mouse brain imaging experiments revealed that enlarged spheroids disrupt action potentials by acting as electric current sinks, leading to neural network dysfunction. Furthermore, larger PAAS sizes and more spheroids per plaque were found in the brain samples of individuals with Alzheimer’s disease compared to those with mild cognitive impairment (MCI), suggesting that both PAAS size and amount may determine the degree of neural circuit disruption and cognitive deficits in Alzheimer’s disease (Figure 3).
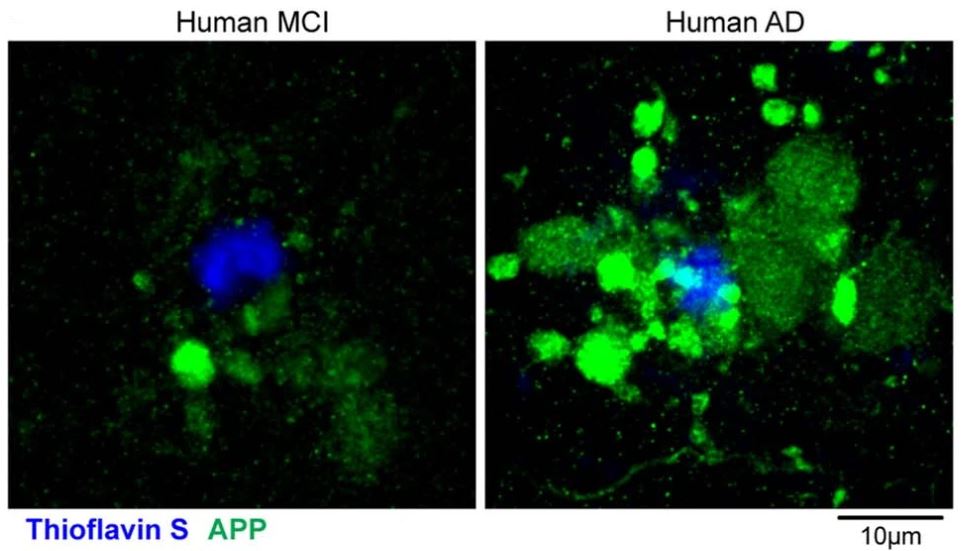
Figure 3: Axonal spheroids in postmortem brain samples from patients with MCI (left) and Alzheimer’s disease (right). © 2022 Yuan et al., licensed under CC BY 4.0.
The authors also found a progressive accumulation of aberrantly enlarged lysosome-associated membrane protein 1-positive vesicles (ELPVs) within axonal spheroids in aging mice. Additionally, small PAASs were found to be predominantly filled with vesicles containing higher levels of cathepsin D, suggesting that spheroid enlargement could be mechanistically linked to ELPV accumulation. Similar findings were observed in postmortem human brain samples, suggesting that ELPV accumulation may drive axonal spheroid enlargement and lead to cognitive dysfunction.
PLD3, which strongly accumulates in axonal spheroids in both humans and mice, was also found to mediate ELPV enlargement and spheroid expansion. Upon deleting neuronal Pld3 in 5xFAD mice by CRISPR-Cas9 gene editing, the authors observed a marked decrease in the abundance of large ELPVs, which was associated with an overall decrease in spheroid size (Figure 4). The authors note that PLD3 reduction can decrease axonal ELPV accumulation and lead to reduced spheroid growth without affecting plaque number or size, as well as restore axonal conduction properties. Reducing axonal spheroids by Pld3 deletion was also found to improve neural circuit function in 5xFAD mice to nearly wildtype control levels.
Conclusions and implications: Modulating PLD3 levels can reverse axonal conduction abnormalities and restore neuronal network function in Alzheimer’s disease, and targeting PAAS formation could also ameliorate neural network abnormalities. Furthermore, since axonal spheroids are prominent in other neurological disorders, this study establishes a framework for investigating their role in conditions beyond Alzheimer’s disease.
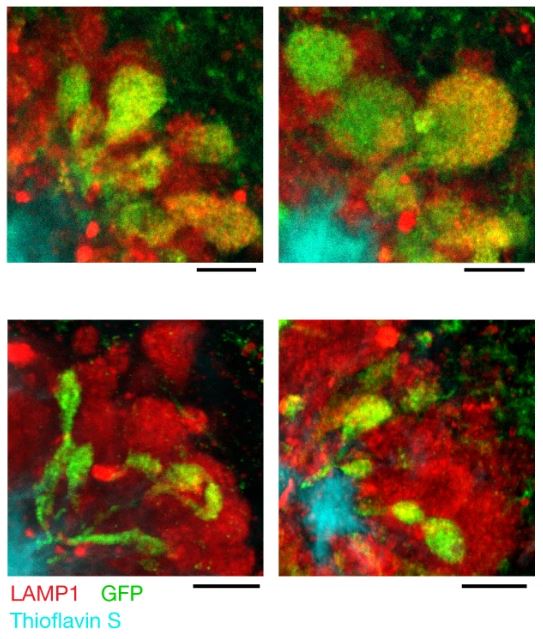
Figure 4: Micrographs of spheroids expressing control (top) or Pld3-targeting single guide RNA (bottom) in mice. Scale bars = 5 μm. © 2022 Yuan et al., licensed under CC BY 4.0.
Perspectives and outlook
Most drug development efforts for Alzheimer’s disease treatment have focused on amyloid plaque removal, but considering alternate pathways could help accelerate drug discovery and development, and ultimately improve the quality of life of those affected by this disease. Based on the present studies, medin and PLD3 represent putative targets for these sorts of diversified novel therapeutic approaches. To learn more about recent advances in Alzheimer’s disease therapies, click here.
About the Author
About the Author

Nina Culum graduated from the University of Western Ontario with a Master of Science in physical and analytical chemistry. During her graduate studies, she fabricated plasmonic nanohole arrays to capture extracellular vesicles and detect cancer by surface-enhanced Raman spectroscopy. Prior to attending UWO, Nina completed her Bachelor of Science in chemistry at the University of Waterloo.
References
- Centers for Disease Control and Prevention [Internet]. Alzheimer’s disease and related dementias; 2020 Oct 26 [cited 2022 Dec 12]. Available from: www.cdc.gov/aging/aginginfo/alzheimers.htm.
- Li X, Feng X, Sun X, Hou N, Han F, Liu Y. Global, regional, and national burden of Alzheimer’s disease and other dementias, 1990–2019. Front Aging Neurosci. 2022;14:937486. DOI: 10.3389/fnagi.2022.937486.
- Kumar A, Nemeroff CB, Cooper JJ, Widge A, Rodriguez C, Carpenter L, et al. Amyloid and tau in Alzheimer’s disease: biomarkers or molecular targets for therapy? Are we shooting the messenger? Am J Psychiatry. 2021;178(11):1014-25. DOI: 10.1176/appi.ajp.2021.19080873.
- Frisoni GB, Altomare D, Thal DR, Ribaldi F, van der Kant R, Ossenkoppele R, et al. The probabilistic model of Alzheimer disease: the amyloid hypothesis revised. Nat Rev Neurosci. 2022;23:53-66. DOI: 10.1038/s41583-021-00533-w.
- Wagner J, Degenhardt K, Veit M, Louros N, Konstantoulea K, Skodras A, et al. Medin co-aggregates with vascular amyloid-β in Alzheimer’s disease. Nature. 2022;612:123-31. DOI: 10.1038/s41586-022-05440-3.
- Yuan P, Zhang M, Tong L, Morse TM, McDougal RA, Ding H, et al. PLD3 affects axonal spheroids and network defects in Alzheimer’s disease. Nature. 2022;612:328-37. DOI: 10.1038/s41586-022-05491-6.