Q&A Report: Cerebral Open Flow Microperfusion (cOFM) for in vivo Cerebral Fluid Sampling – Comparison of cOFM and Microdialysis
The answers to these questions have been provided by:
Deputy Head of Biomedical Tissue Monitoring.
HEALTH – Institute for Biomedicine and Health Sciences
JOANNEUM RESEARCH Forschungsgesellschaft m.b.H
and
Lab Head
In vivo Neuroscience Pharmacokinetics
AbbVie
Do you need to do any purification of the sample while using cOFM?
Whether cOFM samples need to be purified depends on the analytical method used for substance measurement.
cOFM allows sampling of substances inside the intact blood-brain barrier (BBB) regardless of their size and lipophilicity. The cOFM sample is a diluted version of the brain interstitial fluid (ISF). cOFM samples include all components of the ISF regardless of their size – from neurotransmitter to proteins – but in a diluted form. Components that could prevent successful substance analysis must first be removed. In our lab at JOANNEUM RESEARCH the sample purification is an integrated step in sample preparation, if this is needed.
Is the guide cannula made of the same material as the cOFM probe?
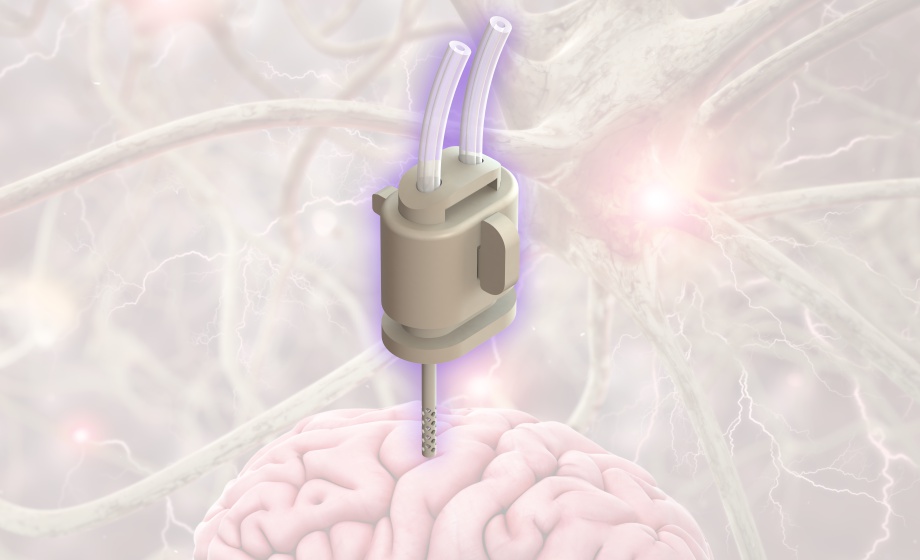
All components of the cOFM probe are made of the same material. The cOFM probe consists of three parts, guide cannula, healing dummy, and sampling insert. The material used has excellent properties in terms of substance adsorption and biocompatibility. However, tubing material is different from probe material.
How about using tritiated water in the push cannula, and see how much reduction of radioactivity in the collection?
This is an interesting approach for in-vivo recovery estimation. However, in our experience the recovery of molecules depends on different factors; e.g. flow rate, direction of net transport, size of molecule, pharmacokinetic inside tissue, blood-brain barrier transport. Therefore, recovery is individual for different classes of molecules. The proposed retro-perfusion approach with tritiated water might work for small hydrophilic substances but will fail for large molecules like antibodies. Therefore, we recommend the zero-flow-rate method for a more reliable in-vivo recovery estimation
What flow rates are used? Since there is no dialysis with cOFM, efficiency shouldn’t be as dependent on flow rate as microdialysis?
Typical flow rates for cOFM sampling are between 0.3 µl/min and 1 µl/min. Similar to microdialysis, cOFM efficiency also depends on the flow rate, but in a different way, since substance transport into the perfusate is not limited by a semipermeable membrane. For cOFM, substrate transport into the perfusate is a combination of diffusion and convection.
Are there any metals in the flow pathway?
No, all parts are made of plastic.
Have you validated your system with drugs that are substrates of P-glycoprotein, with and without a P-glycoprotein inhibitor?
This an interesting approach. However, we used different methods for validation of blood-brain barrier (BBB) integrity during cOFM sampling. The BBB integrity during cOFM sampling was validated by using sodium fluorescein (Naf) and Evans Blue as BBB integrity marker. Both approaches showed the intactness of BBB during cOFM sampling compared to control animals. Birngruber et al. 2013 (10.1111/1440-1681.12174)
What are the advantages of microdialysis over cOFM?
The microdialysis sample represents a filtered and diluted version of the brain ISF, with the filter size defined by the cut-off of the semipermeable membrane. Large molecules or molecules with a high adsorption tendency can thus be excluded from the microdialysis sample. In contrast, the cOFM sample represents an unfiltered diluted version of the brain ISF containing all components of the ISF.
How are the antibodies developed, are they commercial?
Antibodies used in the presented study are commercially available.
Is there any possibility that the probe can absorb tissue instead of interstitial fluid?
No, the cOFM system works with push-pull pump mode. Perfusate is pumped into the probe and withdrawn with the same flow rate from the probe. The cOFM sample is generated by two
mechanisms; diffusion of substances into the perfusate and convective mixing of ISF and perfusate. A special pump tubing assembly ensures that both paths (in and out flow) always work
synchronous
Is it possible to perform cOFM CSF sampling in mice as well? Or is it only possible in rats?
Currently this is not possible, since the outer diameter of the cOFM probe (OD=0.5 mm) exceeds the dimensions of the mouse ventricle. However, we are aware of that drawback, and we are currently developing a smaller version of the cOFM probe that can also be used for CSF sampling in mice.
Can you control things so that the BBB does not open during the experiment?
We have shown that the BBB is intact 14 days after cOFM probe implantation and that it stays intact during the cOFM sampling procedure. We are aware of the different factors that might comprise BBB integrity (e.g. osmotic opening). Therefore, our perfusate is designed to match the osmolarity of plasma and flow rates are kept low to reduce shear forces by the perfusion fluid. However, in some rare cases BBB integrity can be weakened by other factors (e.g. inflammation). To also cover these rare cases, we have developed a method to monitor BBB integrity throughout the whole sampling procedure. This allows a post-hoc analysis of BBB integrity and identification of compromised samples.
Can you really measure any substance in the brain, is there no adsorption?
cOFM probe and system components were selected to minimize non-specific adsorption. However, non-specific adsorption can never be completely excluded. Therefore, we recommend
performing an in-vitro adsorption test prior to any in-vivo experiment. This provides information on the adsorption of the substance in question. In addition, adsorption to the material of the system can be reduced by adjusting the perfusate composition (e.g. by adding bovine serum albumin).
Is it also possible to deliver substances with the cOFM into the brain?
Yes, this is possible either by adding a substance to the perfusate or by direct intracerebral injection via the cOFM probe.
To use a flow meter, do you need to add any chemical in perfusate?
No, you do not need to add any additional chemicals for flow measurement.
Is there the possibility that the probe is broke down during the probe implantation? Which is the success rate?
This is very unlikely and has never happened in any of our experiments. cOFM probe material is shock resistant in contrast to the fragile microdialysis membranes. Moreover, the healing dummy protects the open exchange structure of the OFM probe from deformation.
Which is the minimum time necessary to collect enough volume to analyze?
Time resolution depends on three factors; the minimal volume needed for analysis, the flow rate and the in-vivo recovery of the analyte. The optimal setup is usually determined in a pilot study.
It is interesting to what extent microperfusion can cover the space around a certain structure, for example, the hippocampus when a substance is applied?
Distribution in the tissue depends on various properties of the substance (solubility, MW, concentration in the perfusate, pharmacokinetics inside the tissue) and on the flow rate of the
perfusate through the probe. Thus, extent can be different for various substances.
We recommend to perform pilot studies to evaluate the extent for each substance that should be applied. In our experience we saw that the delivery of a small ionic substance was approx. 3 times higher when applied via cOFM perfusion compared to microdialysis.
Do you have an information about a stability of collected cOFM samples that later stored in - 80°C?
Sample stability should always be tested for individual substances. In general, stability of cOFM samples is similar to other biological samples stored at -80°C.
Roughly, how much cISF volume do you get after ~7 days of collection?
cOFM probe works on the principle of perfusion, therefore no pure cISF is withdrawn from the tissue. The perfusate is in direct contact with cISF at the tip of the cOFM probe. Substances present in cISF diffuse into the perfusate, which corresponds to diluted cISF. The sample volume depends on the flow rate of the perfusate through the cOFM probe. Example: With a flow rate of 1µl/min you will collect 60 µl of diluted cISF per hour. This sums up to 7d * 24h * 60 µl = 10 080 µl of diluted cISF in 7 days.
I was wondering if it is possible to use the OFM system to use the skin and for how long the probe can remain in place?
Yes, we have a CE-certified dermal and adipose OFM version that is used in ex-vivo skin explants, in in-vivo preclinical animal models and in humans in clinical studies. The probes are currently approved for use in human for a duration of 48 h, but we have applied for approval to use them up to 3 weeks in humans. For further information you can visit our website: www.openflowmicroperfusion.com.
What age rodents is this technology safe for? Is recovery over that 2-week period the same if used on an age where the brain and/or body continues to grow?
We recommend using cOFM in adult animals, since probe placement might be inaccurate, and the desired brain region might be missed if cOFM is implanted in growing animals. As we are using adult rodents in our studies, we have no information on BBB integrity and recovery of growing animals.
As sampling can be done intermittently over time - what is the longest period that has been done? For example, could sampling be done at one month intervals over a 6 month period? a couple of days but once per month for 6 months?
We successfully did intermittent cOFM sampling in rats over 21 days. The longest implanted cOFM probe stayed in place for 30 days without causing morphological tissue reactions. We have tested the fixation of the probe to the skull for up to 9 months, but we have no morphological and functional data for time points beyond 30 days.
What are the considerations that are different for use in large species? Does anything change?
From a technical perspective, cOFM sampling in large species requires longer probes. cOFM probes of 1 cm length were successfully used in NHP. However, our data set on cOFM sampling in large species is still on a pilot level. Currently we do not have standard probes for large species in our program, but they can be produced as customized probes.
Can probe/cannula have effect on brain distribution of antibody?
It is unlikely that the cannula itself might have an effect on brain distribution. However, the sampling procedure might affect the distribution to a low degree, since substance (e.g. antibody) is constantly removed from the ISF by the probe. This holds also true for microdialysis and has to be kept in mind when interpreting data
How about group housing after surgery?
We recommend that animals are housed individually after probe implantation to avoid probe damage due to social grooming. However, if the probe can be covered after it was implanted, group housing could be an option.
Have you used this in neonatal rodents?
We haven’t used the cOFM probe in neonatal rodents. To allow sampling in the brain with an
intact blood-brain barrier, a 14-day recovery after probe implantation is recommended. Neonatal
rodents have a steep growth curve, which might result in a displacement of the probe tip from the
initially planned brain region. Moreover, the outer diameter of the cOFM probe is 0.5 mm, which
might be inappropriate for neonatal rodents.
Do you have experience with this apparatus for traumatic brain injury models?
Yes, we have experience with TBI and other animal models.
Do we send animals to your facility get measurement done?
Yes, the institute HEALTH provides a contract research service for PK/PD assessment with rodents. Our services include study plan development, study conduction, bioanalytical measurement and support with the interpretation of data. Depending on your scientific question we develop an appropriate study design. Transgenic or wild type animals are purchased from accredited vendors. The bioanalysis of samples is done in our in-house bioanalytical laboratory. We consider ourselves
as one-stop-shop for PK/PD studies in rodents. For more information please contact cofm-services@joanneum.at
Is it possible to measure vesicles from cOFM?
Basically, any substance or structure that is freely present in the interstitial fluid (ISF) can be sampled by using the cOFM probe. Intracellular substances and tissue-bound substances (e.g. to the cell surface) cannot be sampled. If the vesicles are freely present in the ISF it is very likely that they can be measured.
How can cOFM support pharmacodynamic studies?
cOFM can be used to sample substances after dosing inside the brain tissue. The drug and any biomarkers can be quantified in the same sample, which allows the simultaneous assessment of pharmacokinetics and phamacodynamics.
Is there any link cOFM and brown adipose tissue?
We are currently running a project to answer this question, but at this moment we don’t have the results yet to answer this question in more detail.
What method is used to quantify neurotransmitter?
Quantification of substances in the brain can be achieved by different methods (e.g. No-Net-Flux). We recommend the Zero-Flow-Rate protocol, where the absolute concentration inside the brain and the in-vivo recovery are assessed.
Is it possible to reuse the probe?
All cOFM probe components are designed for single use. All parts are gamma sterilized and packed in sterile packages. We do not recommend reusing the probe or its components.
. Please explain about pore size of functional part of probe?
The exchange area of the cOFM probe has macroscopic openings instead of pores. Thus, the perfusion fluid is in direct contact with the brain tissue that surrounds the probe. The length of the
exchange area is either 1 mm or 2 mm for standard probes. However, longer exchange areas are available as custom made probes.
As I know CMA has entered the clinic to monitor patients' brain substance, so will your device move to such implementation?
Currently we are in an early development phase for clinical cOFM. Moreover, we are working on the possibility of bringing clinical cOFM to the market. First in-vivo tests are in progress.
So such cOFM device can be used to deliver some drugs (such as siRNA or others) into target brain sections, then monitor other substances induced by the drug delivery?
Yes, cOFM has no limitations regarding the substance that should be delivered or measured. Both, delivery from the perfusate into tissue and sampling from the tissue into the perfusate are always running simultaneously, a delivery/monitor setup is possible.
While the neutrotransmiter is vital for the brain function, will cOFM be coupled with online MS detection system?
This depends on the sample purification and preparation steps needed prior to MS detection. cOFM sample contains diluted interstitial fluid (ISF), which means that in addition to neurotransmitter there are also enzymes, proteins, antibodies etc. found in the sample. In contrast, microdialysis provides filtered and diluted ISF, where many components of the ISF are
excluded by the semi-permeable membrane.
You mentioned that cOFM could be used to monitor awake animals, so concerning the tubing outside, will the animal be tested in single?
Animals are housed individually in special awake animal sampling cages (e.g. Raturn/Culex, BASi) during the cOFM sampling procedure. This system avoids tangling of tubing and allows safe and automated sampling at a reduced stress level for the animals.
. Have the probes been used in aged animals, like more than 2 years old? Does it cause more trauma to brain tissue compared to animals less than 1 year old?
Usually, animals used in our studies are adult (up to 12 months), depending on the scientific questions. However, trauma healing in aged animals is reduced, we thus recommend to extend
post-surgical trauma recovery to allow the blood-brain barrier to re-establish.