Q&A Report: Microvascular & Functional Ultrasound Imaging: Insights into Stroke and Neurovascular Diseases
These answers were provided by:
Franck Lebrin, PhD
Associate Professor
Internal Medicine
Leiden University Medical Center
Denis Vivien, PhD
Professor/Hospital Practitioner
Cell Biology
Univ. Caen-Normandy
Do you think it is possible to reach higher resolution using ultrasound localization microscopy (ULM)?
Franck Lebrin: Yes, but in order to detect smaller blood vessels, you need to detect more gas microbubbles and both the concentration of microbubbles in the blood circulation and the time needed to record and accumulate enough microbubbles need to be adapted.
How do you expect to translate your preclinical results to clinical practice using fUS?
Franck Lebrin: In humans, the skull remains an issue if you want to image the full brain but acquisition of large field of views by the temporal region is possible and then specific brain regions responding to external stimuli could be defined. Alternatively, the retina could also be an option for fUS, as many people with neurological disorders also show defective vascular responses in the retina. We are currently testing fUS in patients with HHT to determine whether we can detect similar biomarkers to those found in mice.
There is still a debate about the different populations of mural cells. Do you have examples of diseases with different mural cell defects?
Franck Lebrin: Yes, many inherited small cerebral vascular diseases have defective mural cells at the arterial-arteriolar capillary sites but do not show thin strand pericyte anomalies whereas Alzheimer ‘s disease, for example, shows thin strand pericyte malfunctions and blood brain barrier leakage.
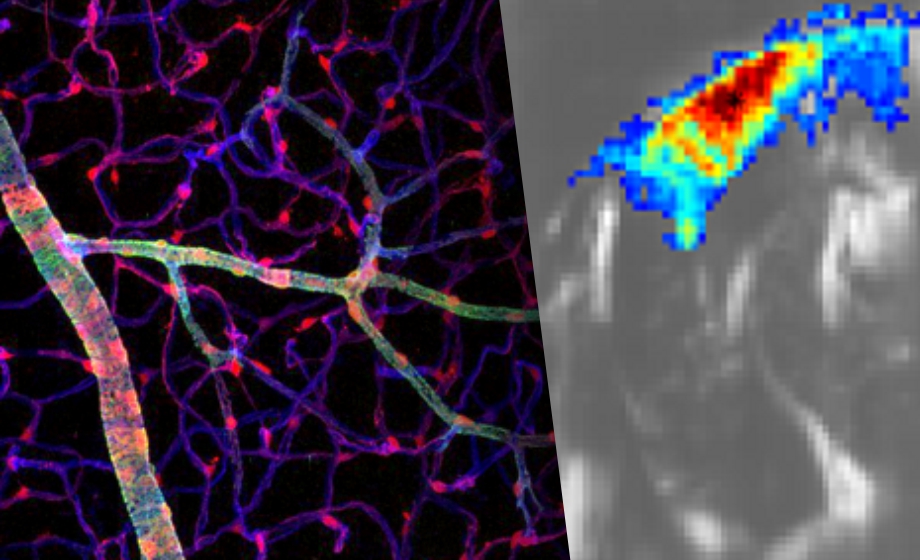
How are you able to form the micro-fiber architecture image using fUS? What model do you use of the fiber arrangement?
Franck Lebrin: I do kindly refer to the paper of Errico et al., 2015 Nature from M. Tanter’s lab that describes ultrasound localization microscopy.
What are the possibilities of translation to clinic of the methodologies presented (MR imaging and fast ultrasound imaging in the field of stroke)?
Denis Vivien: We are close to clinical application. The group of M. Tanter and colleagues have already demonstrated its feasibility to image the human brain. Our group is working on this, with a human POC planned for the coming months.
In your opinion, what are the gaps that need to be filled in prior to translation to the clinic for fast ultrasound imaging?
Denis Vivien: The gap is to convince clinicians of the relevance of this modality and that it will provide the same level of information as CT-Scan or MRI. One remaining limitation is access to the whole brain.
You mentioned that fast ultrasound imaging could be used to predict responses to treatment. What would be the added value in clinical practice?
Denis Vivien: As we demonstrated, the fUS can be used to predict very early after treatment with fibrinolytics the response to the drug, i.e. recanalization efficacy. This is critical to optimize the use of fibrinolytics.
How applicable could fast ultrasound imaging be for the early detection of vasospasm in cases of sub-arachnoid hemorrhage?
Denis Vivien: This is not demonstrated so far. But it is an exciting challenge. We think that it could work. If it works it would be an important added value in clinic to prevent or cure delayed cerebral ischemia after intracerebral hemorrhages.
Could you repeat your stroke model findings in awake mice?
Denis Vivien: Yes, it is working now. Not yet published but should be very soon.
Did you require constant rate infusion of SonoVue to form the awake images or just a bolus?
Denis Vivien: A bolus is fine, it depends how long you would like to image the brain at very high resolution.