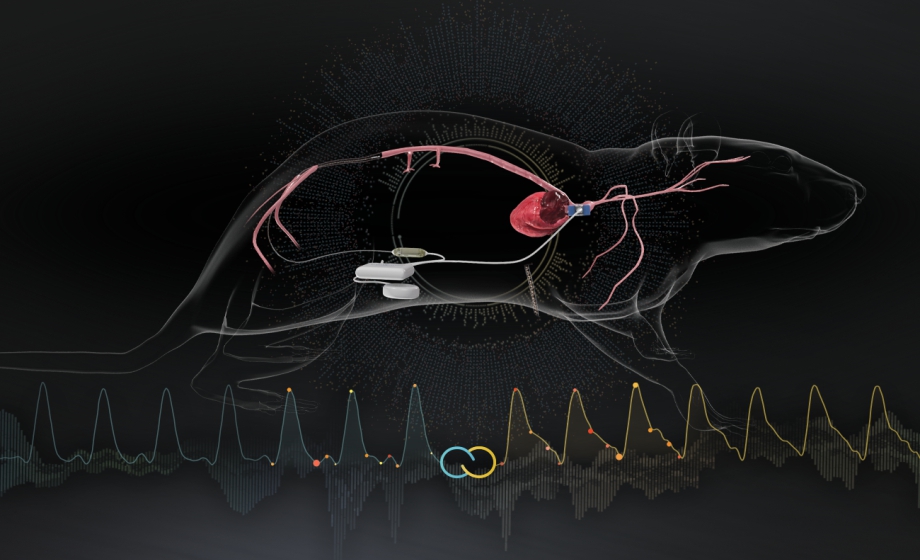
Q&A Report: Untether Your Data with EndoGear: Wireless Volumetric Blood Flow and Pressure Measurement
Does development of connective tissue between vessel and probe affect the doppler signal?
No. The connective tissue growth actually helps stabilize the preparation and acoustic coupling between vessel and probe while allowing for animal/vessel growth and maintaining signal accuracy.
Are there other sensor types like ECG or blood oxygen available?
As of today, blood flow and blood pressure are our featured sensors. However, we are always interested in improving our equipment, and have additional sensors planned for future. You can stay in touch with us by visiting our website.
Has anyone used the pressure recording technology in the gastrointestinal tract?
We are not aware of anyone using EndoGear® in this application, currently.
Has anyone worked with smaller animals than 250g?
Yes, in some studies, animals weighing 215- 220g were implanted with success.
I am currently measuring tethered cardiac output with your equipment, what are the surgical differences in tunneling with EndoGear® in regards to the flowprobe?
Tethered preparations usually have equipment exteriorized through the scapular area. Since all sensors are hardwired to the EndoGear® Implant, the tunneling route for any sensor is from implant location to the target vessel of that specific sensor. For example, when using implants to be placed in the abdominal area, the flowprobe to be placed on the ascending aorta must be tunneled form the abdomen to the chest. Please visit our Applications section of the EndoGear® webpage, for more information.
I have not measured flow before with your technology, is that a problem?
Not a problem at all! We have been working closely with researchers helping develop surgical protocols for decades. We have a full suite of resources, training options and staff to help you establish a new procedure in your protocol.
I’m working with mice, will EndoGear® be offered for mice? What about large animals like pigs?
EndoGear® is not currently available for mice. EndoGear® for large animals is currently in development, please check back with us on this in the coming months.
If using the inductive power system, can the animal be taken out of the base to record data?
No. The inductive power system provides continuous power to animals while they are caged, only.
Can you reuse/resterilize the EndoGear® implant?
Yes, the implant can be reused. For implants that rely on a battery, the usable life span of the implant will be dictated by the battery life, however this constraint is not present for implants configured for wireless power technology. Reusability is also directly related to the success of the explantation procedure itself and relies on the user’s ability to not damage the implant, cables, and sensors.
EndoGear® Implants are compatible with Ethylene Oxide sterilization method, under cold cycle with 37°C exposure.
We provide complete training for EndoGear®, including explantation procedures.
The data you presented was mostly focused on the cardiovascular system, can it be used in stroke models measuring carotid flow?
Yes, absolutely. However, the type of stroke model needs to be evaluated for suitability. As an example, stroke models induced by mechanical carotid stenosis may have coils or other types of devices placed around the vessel that are incompatible with the proper functioning of the flowprobe. We would suggest a discussion with our Application Team to review your needs.
Is it possible to place the flowprobe in a different blood vessels to measure flow for example in mesenteric artery?
Yes. Superior mesenteric artery blood flow has been measured with our 1.5 and 2.0 mm flowprobes. Our implants can be configured with the following flowprobes sizes:
- 1mm
- 1.5 mm
- 2 mm
- 2.5 mm
- 3 mm.
What is the accuracy and resolution of the pressure sensor of Scisense catheter?
Please refer to our Scisense pressure catheter data sheet.
Can implant bodies also be placed subcutaneously?
Yes. We recommend subcutaneous placement on the flank/dorsal area.
How fragile is the new solid-state catheter?
This is a common question regarding solid-state catheters. We manufacture our Scisense catheter to be as robust as possible, to resist kinking, and incorporate appropriate strain relief to give flexibility when needed. With this said, no catheter design is impervious to damage. Handling/care will be important to preserve its lifespan.
How robust are implants to fluid leaks?
The implants are made to be impervious to fluid ingress. We have designed a complex material structure to provide the best possible outcome while providing biocompatibility and minimizing animal discomfort. Our sensors are designed under similar principles.
With regard to post-experiment data analysis, does your support department provide assistance with measurement calculations to enhance our research questions?
Yes. We have been providing this service for our pressure-volume loop equipment, both in academia and industry, for many years. We would welcome the opportunity to help you optimize your calculations with EndoGear®.
What are the measurements of the power supply that the cage sits on? We do have some height limitations in our cage racks.
The wireless power supply dimensions are: length 635 mm x width 313.8 mm x height 80.1 mm.
Weight 4.095 kg.
Weight of Cage Lid 1.533 kg.
Weight of Cage and Cage Lid 2.896 kg.
Total weight with cage and lid 6.991 kg.
Total height ~240cm when all components are assembled.
For more information related to the Wireless Power Supply, click here.
Our system is designed to operate on a plastic rack. With our setup, multiple cages can be placed on a single plastic rack, 36” x 23” x 72”.
Can this device measure the cerebrospinal fluid pressure? Can it measure the blood pressure and cerebrospinal fluid pressure simultaneously?
Cerebrospinal fluid pressure ranges from ~2.3 to + 6.2 mmHg. Our pressure sensors are sensitive enough to provide this resolution, yes. However, this specific application is not one that we have experience with using EndoGear®. Contact our Application Team if you would like to discuss this further.