The Cancer-Immunity Cycle: Research Solutions for Preclinical Immuno-Oncology
Blog post by Nina Culum, MSc
Cancer immunotherapy has undoubtedly expanded the cancer treatment landscape, improved patient outlook, and is now a pillar of cancer care [1]. Such progress can in part be attributed to the recognition of the importance of the cancer-immunity cycle as a whole [2]. Factors in the tumor microenvironment (TME) can modulate activated antitumor immune responses, emphasizing that the cancer immune response actually comprises a series of distinct and regulated steps that are optimally addressed as a group [3]. By considering the cancer-immunity cycle as a whole, more effective and novel immunotherapeutic targets and mechanisms can be identified. But first, what exactly is the cancer-immunity cycle?
Overview of Crown Bioscience’s immuno-oncology research approaches and assays.
The cancer-immunity cycle: a brief overview
Described in detail by Chen et al., the cancer-immunity cycle refers to a sequence of events that must be initiated and propagated for the anticancer immune response to result in cancer cell death (Figure 1) [3]. Briefly, neoantigens are released and captured by dendritic cells, which must be accompanied by signals that specify immunity for an anticancer T cell response. Dendritic cells then present captured antigens to T cells, leading to priming and activation against cancer-specific antigens; this step determines the nature of the immune response, with the balance of T effector and regulatory cells being critical to the final outcome. Lastly, activated effector T cells traffic to and infiltrate the tumor bed, recognize and bind to cancer cells, and kill their target cancer cell; this releases more tumor-associated antigens, thus beginning the cycle anew.
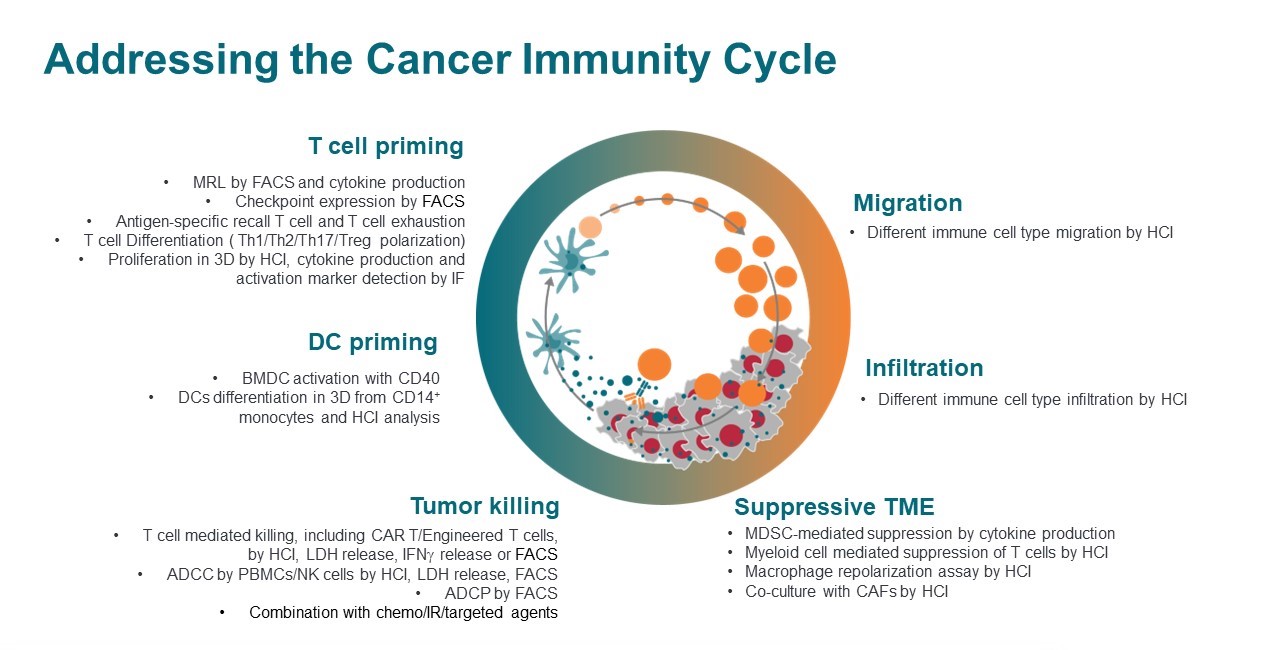
Figure 1: Illustration of the cancer-immunity cycle, adapted from ref. 3.
In cancer patients, tumor antigens may go undetected, dendritic and T cells may not treat antigens as foreign, T cells may be inhibited from tumor infiltration, and factors in the TME may suppress effector cells that are produced [3]. For cancer immunotherapy treatments to be effective, they must retain activity despite negative feedback mechanisms, which therefore requires consideration of the cancer-immunity cycle as a whole. A number of innovative and efficient techniques are now available to researchers for preclinically screening new treatment approaches, with the choice of appropriate model depending on the purpose of the study. For this blog, we’ll be highlighting some in vitro and in vivo models that facilitate cancer immunotherapy research, including organoids, xenografts, and genetically engineered mouse models (GEMMs).
In this series, experts from Crown Bioscience will review how cancer immunotherapy is studied along each step of the cancer-immunity cycle, including an overview of technologies, techniques, challenges, and emerging research questions to be addressed. REGISTER NOW
Organoids
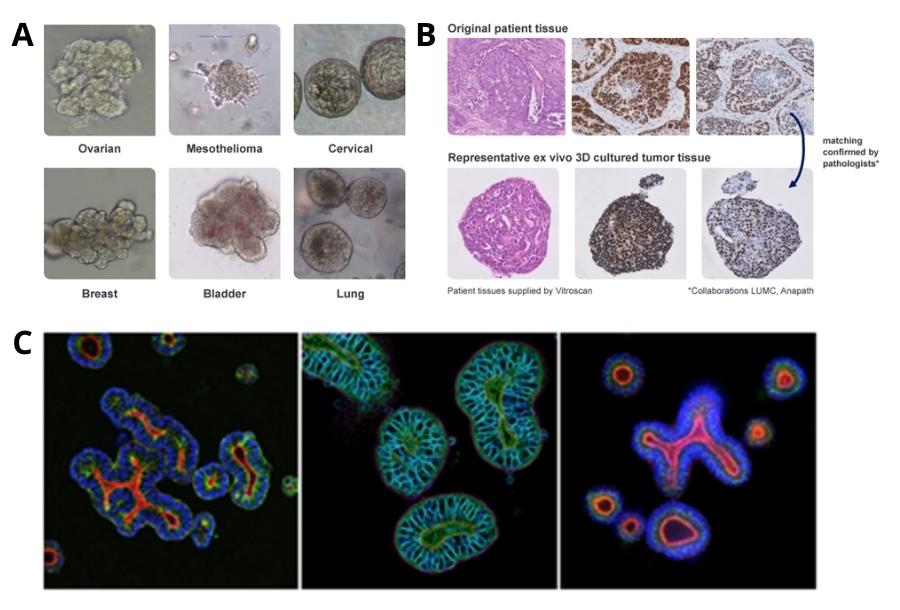
When embedded into a 3D matrix, tissue-derived adult stem cells can grow into self-organizing organotypic structures known as organoids, which have been established from a wide range of cancer tissues (Figure 2A-B) [4]. In vitro 3D culture technologies such as organoids have facilitated the development of novel, physiologically-relevant human cancer models, which are essential for translation of preclinical research into effective cancer treatments. Importantly, organoids can be expanded long-term, cryopreserved, and genetically modified, making them useful for a range of applications including basic research (e.g., interrogating mutational processes and cancer development) and translational research (e.g., drug development and personalized medicine) [4]. Additionally, researchers who use organoids in their studies can employ high content imaging (HCI) to obtain valuable insights into complex disease pathophysiology and assess novel therapies (Figure 2C).
Figure 2: (A) Bright field images of different organoid indications highlighting their morphology. (B) Comparison between patient biopsy samples (top) and corresponding organoids (bottom), demonstrating great similarity which has been confirmed by clinical pathologists. (C) Colorectal cancer organoids analyzed by HCI.
Cell line- and patient-derived xenograft models
Rodent models of cancer and the pharmacodynamic/pharmacokinetic information they provide are critical to understanding cancer pathophysiology, identifying new targets and therapies, and exploring mechanisms of drug resistance [5]. Xenografts in particular are the most widely used in vivo model for investigating tumor growth rates and metastasis, and can further be subdivided into cell-derived xenograft (CDX) and patient-derived xenograft (PDX) models [6]. CDX models are produced by subcutaneously injecting cancer cell lines into immunodeficient mice, which is a simple process resulting in comparatively rapid tumor formation [7]. One limitation of CDX models, however, is that they do not represent the tumor heterogeneity of individual patients and therefore may not accurately predict the drug response of the original tumor of interest [6]. In contrast, PDX models are generated by implanting cancerous tissue from patients into mice, thus maintaining tumor histopathology and genetics at the trade-off of higher maintenance costs [6, 7].
Humanized PDX models
Although PDX models can be excellent tools for chemotherapeutic drug studies, an intact immune system is required for assessing cancer immunotherapies. Therefore, mice with human immune systems (i.e., “humanized mice”) are necessary for screening immunotherapeutics [8]. Humanized mouse models are produced by implanting human hematopoietic stem cells, tissues, or lymphocytes into immunodeficient mice, from which humanized PDX models can be generated upon implantation with fresh human tumor fragments [7, 8]. In addition to its applications in cancer immunotherapy research, humanized PDX models have also been used to study interactions between tumor and immune cells in the TME [8]. Models like MiXeno also offer an alternative, simpler solution to the full stem cell reconstitution approach while still providing a unique opportunity for studying immunotherapies within a human TME.
Genetically engineered mouse models
In addition to CDX and PDX models, GEMMs are also commonly used in immuno-oncology research. Tumorigenesis in GEMMs is induced by promoting oncogene expression or deleting tumor suppressor genes by genetic engineering [7]. Unlike xenograft models, GEMMs form orthotopic tumors in immune-proficient microenvironments, thereby simulating the tumorigenesis process. However, these models cannot accurately predict interactions between the tumor and human immune system [7]. Many drug candidates that exhibit good therapeutic efficacy at the preclinical stage do not translate well into clinical practice, further highlighting the need for animal models that have humanized immune systems, such as HuGEMM, which are engineered to express humanized drug targets (e.g., genes encoding for immune checkpoint proteins).
Perspectives and outlook
As each in vitro and in vivo cancer model has its own advantages and limitations, the choice of model used in cancer immunotherapy research should be selected with the study design and rationale in mind. For example, certain systems are well-suited for high-throughput preliminary screening of compounds of interest, while others are more appropriate at later stages of drug discovery. Crown Bioscience in particular offers many different cancer model systems and services to support drug development in the discovery, preclinical, or translational phase. For a deep dive into the cancer-immunity cycle, T cell biology, suppressive TME compartments, and advanced models and techniques for assessing cancer immunotherapeutics, click the button below to sign up for Crown Bioscience’s webinar series!
About the Author
About the Author

Nina Culum graduated from the University of Western Ontario with a Master of Science in physical and analytical chemistry. During her graduate studies, she fabricated plasmonic nanohole arrays to capture extracellular vesicles and detect cancer by surface-enhanced Raman spectroscopy. Prior to attending UWO, Nina completed her Bachelor of Science in chemistry at the University of Waterloo.
References
- Esfahani K, Roudaia L, Buhlaiga N, Del Rincon SV, Papneja N, Miller WH. A review of cancer immunotherapy: from the past, to the present, to the future. Curr Oncol. 2020;27(s2):87-97. DOI: 10.3747/co.27.5223.
- Peterson C, Denlinger N, Yang Y. Recent advances and challenges in cancer immunotherapy. Cancers. 2022;14(16):3972. DOI: 10.3390/cancers14163972.
- Chen DS, Mellman I. Oncology meetings immunology: the cancer-immunity cycle. Immunity. 2013;39(1):1-10. DOI: 10.1016/j.immuni.2013.07.012.
- Drost J, Clevers H. Organoids in cancer research. Nat Rev Cancer. 2018;18:407-18. DOI: 10.1038/s41568-018-0007-6.
- Ruggeri BA, Camp F, Miknyoczki S. Animal models of disease: pre-clinical animal models of cancer and their applications and utility in drug discovery. Biochem Pharmacol. 2014;87(1):150-61. DOI: 10.1016/j.bcp.2013.06.020.
- Khan AQ, Siveen KS, Prabhu KS, Kuttikrishnan S, Akhtar S, et al. Role of animal research in human malignancies. In: Azmi A, Mohammad RM, editors. Animal models in cancer discovery [Internet]. Elsevier Inc.; 2019 [cited 2022 Jul 08]. pp. 1-29. DOI: 10.1016/B978-0-12-814704-7.00003-9.
- Li Z, Zheng W, Wang H, Cheng Y, Fang Y, et al. Application of animal models in cancer research: recent progress and future prospects. Cancer Manag Res. 2021;13:2455-75. DOI: 10.2147/CMAR.S302565.
- Choi Y, Lee S, Kim K, Kim S-H, Chung Y-J, Lee C. Studying cancer immunotherapy using patient-derived xenografts (PDXs) in humanized mice. Exp Mol Med. 2018;50(8):99. DOI: 10.1038/s12276-018-0115-0.