In this webinar, Professor Edward Bahnson describes a traditional rat carotid balloon injury model to study arterial restenosis and presents a novel methodological approach involving 3D imaging of vascular injury models.
Highlights
- How nuclear factor-erythroid factor 2-related factor 2 (Nrf2) affects the redox status of cells
- The effects of cinnamic aldehyde treatment on Nrf2
- Evidence for a Nrf2-dependent mechanism of neointimal hyperplasia inhibition by cinnamic aldehyde treatment
- Benefits of 3D analysis of arterial injury over traditional 2D histology analysis
Webinar Summary
Atherosclerosis is the leading cause of death and disability worldwide, and its prevalence is expected to increase with aging populations. Treatment options for severe atherosclerotic disease involve percutaneous or surgical revascularization. However, long-term treatment success is limited by the development of arterial restenosis, which is driven by neointimal hyperplasia and vessel remodelling. Herein, Professor Bahnson utilizes a rat carotid balloon injury model to study arterial restenosis and presents a novel approach to analyze vascular injury by 3D imaging.
“My lab studies the rat carotid balloon injury model, which is what I refer to as an “old dog” because it’s been around for a long time.”
Restenosis is driven by redox dysfunction, where vascular injury leads to activation of NADPH oxidase 1 and produces reactive oxygen species (ROS). Smooth muscle cells then migrate and proliferate, resulting in neointimal hyperplasia. Since redox dysfunction drives neointimal hyperplasia, the Bahnson group selected a target that could affect the redox status of cells: nuclear factor-erythroid factor 2-related factor 2 (Nrf2). In the presence of electrophiles or oxidative stress, the Nrf2 inhibitor undergoes a conformational change. No longer a target for proteasomal degradation, Nrf2 can accumulate in the cytosol, translocate to the nucleus, and drive the transcription of antioxidant enzymes and other cytoprotective genes.
The Bahnson group utilizes cinnamic aldehyde as an electrophile for Nrf2 activation. Key findings suggest that cinnamic aldehyde not only increases levels of enzymes that regulate redox homeostasis, but also their activity. Cinnamic aldehyde also reduces vascular smooth muscle cell (VSMC) numbers without inducing cell death, thereby inhibiting smooth muscle cell migration and proliferation. By assessing biomarker levels, the Bahnson group further demonstrated that cinnamic aldehyde treatment at the time of injury reduces the local oxidative burden within VSMCs as well as immune cell infiltration. Furthermore, the co-localization of Nrf2 with VSMC nuclei was observed only after treatment, suggesting that cinnamic aldehyde activates Nrf2.
“We have an activation of the pathway in vivo, decrease in oxidative stress markers, and decrease in inflammation. Does this result in a decreased proliferation and decreased neointimal hyperplasia?”
Indeed, a decrease in the proliferative index of the vascular walls was observed following cinnamic aldehyde treatment, resulting in neointimal hyperplasia inhibition two weeks post-injury. However, to investigate whether neointimal hyperplasia is Nrf2-dependent, the Bahnson group introduced a Nrf2 knockout (KO) model with which to compare cinnamic aldehyde effects. VSMC migration and proliferation were inhibited only in the wildtype (WT) animals, suggesting that there is a Nrf2-dependent mechanism for neointimal hyperplasia. However, biomarker levels were decreased in both animal models, albeit to a larger extent in the WT model than the KO model. Decreased biomarker levels in KO animals suggests that there could also be a Nrf2-independent manner by which cinnamic aldehyde results in decreased superoxide levels, but not enough to observe an effect on migration or proliferation.
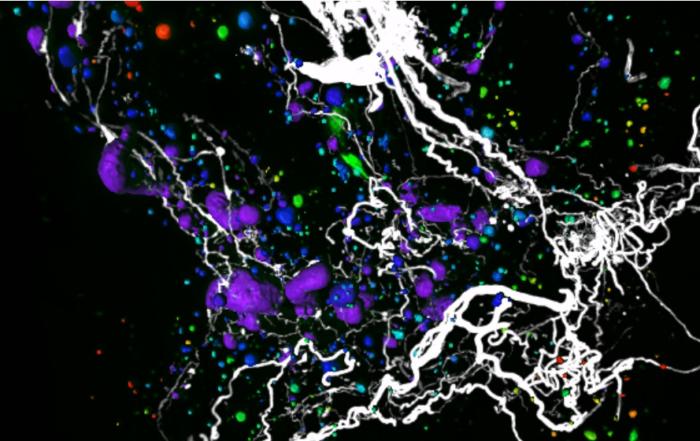
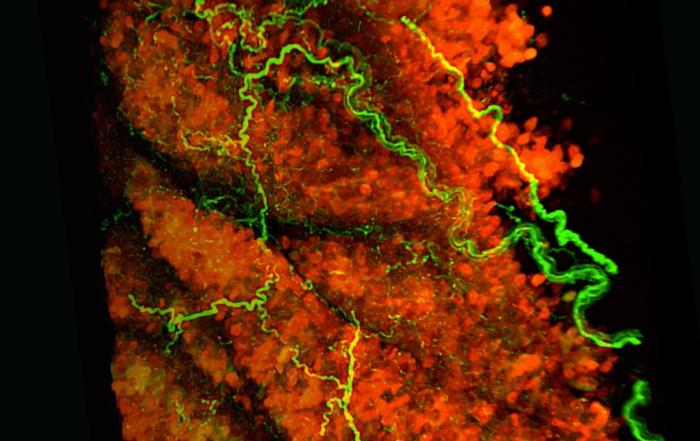
Professor Bahnson next discusses the advantages of using a 3D model over a classic 2D model. 2D datasets intrinsically contain less information than 3D datasets, and so important data could potentially be excluded. Since 2D analysis requires physical sectioning and selection by the researcher, analysis is quite time-consuming and tedious, and user bias is inevitable. Moreover, the lack of standardization and reproducibility in 2D analysis has been acknowledged by researchers in this field. Therefore, the Bahnson group employed light sheet fluorescence microscopy (LSFM) to image preclinical vascular injury models in 3D, thereby increasing analysis efficiency while minimizing bias.
When comparing the intima:media ratio of carotid arteries calculated by the optical volume from LSFM to traditional hematoxylin and eosin (H&E) staining, no significant difference was found between the two methodologies. Professor Bahnson therefore deduces that the parameters used in this novel technology are the same as the parameters that have been used and validated in literature for decades. However, the optical volume calculations obtained using LSFM yielded a much smaller coefficient of variation than those from H&E staining, suggesting that 3D analysis is more precise than 2D analysis.
Using Nrf2 KO and WT models once again, the Bahnson group demonstrated that cinnamic aldehyde inhibits neointimal hyperplasia in a Nrf2-dependent manner, as neointimal hyperplasia volume decreased significantly after treatment only in WT rats. The 3D approach not only allowed for imaging of the entire hyperplastic lesion, but also allowed for the assessment of compensatory and constrictive remodelling.
Cinnamic aldehyde was also found to induce compensatory remodelling by a Nrf2-dependent mechanism, but did not affect constrictive remodelling. Due to the additional accurate remodeling quantification offered by 3D analysis, Professor Bahnson notes that 3D analysis can even drive scientific discovery in a way that 2D analysis cannot. The novel methodology presented in this webinar could pave the way for more precise, efficient, and advanced analysis of carotid artery injuries in the future.
Click to watch the webinar recording. To view the presentation full screen simply click the square icon located in the bottom-right corner of the video viewer.
Resources
Q&A
- Could cinnamic aldehyde be applied to atherosclerotic vessels in the future to reduce ROS and atherosclerotic plaque growth?
- Why is 100 μM cinnamic aldehyde used?
- How small of a vessel can be imaged in 3D?
- Is indiscriminate quenching of ROS through Nrf2 activation expected in a therapeutic context?
- Was an in vivo subchronic treatment attempted?
Presenters
Assistant Professor
Department of Cell Biology and Physiology
University of North Carolina at Chapel Hill