In this webinar, Paul Gallant and Edward LeCluyse, PhD, present a novel hepatic tri-culture system that can recreate native liver tissue structure and morphology.
Highlights
- Overview of an all-human hepatic triculture model
- Pharmacological and toxicological applications of the pre-qualified hepatocytes
- Advantages of the triculture system compared to traditional cultures
Webinar Summary
Paul begins this webinar with a brief discussion of some of the current challenges in preclinical translational research, and how LifeNet Health LifeSciences is finding a solution. To date, most preclinical research utilizes animal models as a substitute for testing directly on human tissue samples. While animal models tend to be more abundant and cost effective, the translation between animal models and human physiology is not always perfect. This problem is compounded when the target tissue is an intermediary for many organ systems, requiring realistic intercellular interactions for the tissue to be an accurate model. While models of particular cell types exist for organs such as the liver, they lack the other cell types needed for appropriate function. To address these challenges, LifeNet Health LifeSciences has developed the Human Hepatic Triculture Model capable of self-organizing to the native environment of the liver.
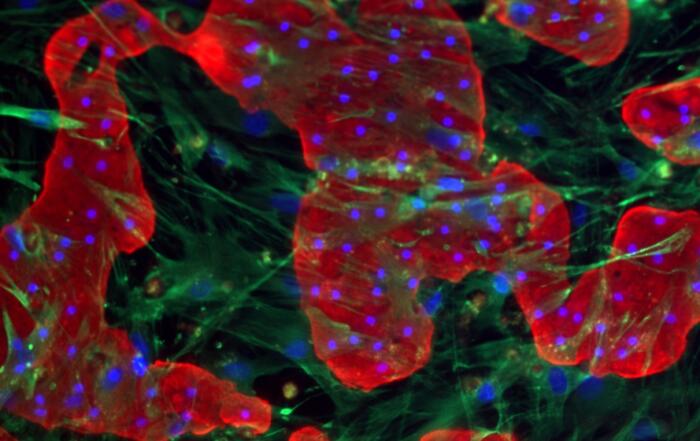
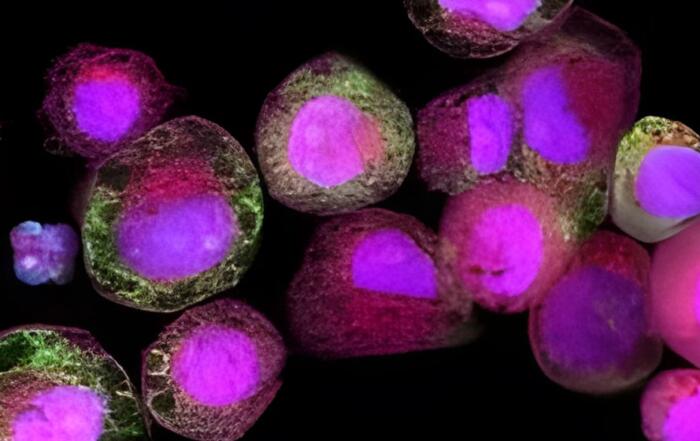
Next, Edward shares how he helped to develop this in vitro human liver model system. The triculture system is derived entirely from human cells and is capable of creating an in vivo-like structure, thus maintaining important cell-to-cell interactions. This system also maintains hepatocyte morphology, function, and metabolic activity for up to 14 days, indicating its robustness.
“We wanted to mimic as much of the native [hepatocyte] morphology, as well as direct and indirect cellular interactions.”
The human hepatic triculture kit will be available in both a 24- and 96-well system, and will contain everything required for growth and maintenance. Assembling this system is a three-step process. After the medium has been brought to standard biological conditions, feeder cells must first be established on the plate before hepatocytes can be introduced. The model is then stabilized approximately five to seven days following plating. The hepatic cell culture can remain stable for at least two weeks, during which a variety of experiments can be performed.
Edward also demonstrates the robustness of this tissue culture by comparing it to sandwich-cultured hepatocyte monolayer, a traditional cell model of liver function. For example, the triculture has displayed improved native intercellular interactions and cell polarity compared to the traditional model. The triculture has also demonstrated superior sustained albumin and urea production compared to the monoculture, further highlighting its functionality. Although this product is not yet commercially available, it has the potential to vastly improve future liver immunology and physiology studies.
Click to watch the webinar recording. To view the presentation full screen simply click the square icon located in the bottom-right corner of the video viewer.
Resources
Q&A
- What makes the Human Hepatic Triculture System so easy to use?
- Are you looking into animal models for the triculture system?
- What are the feeder cells, can they be passaged, and is there sequencing data on the donors?
- How often does the media need to be changed?
- Should evaporation be a concern when using the plates for multiple weeks?
- Have you used CYP and UGT inhibitors on the model?
To retrieve a PDF copy of the presentation, click on the link below the slide player. From this page, click on the “Download” link to retrieve the file.
Presenters
Chief Scientist
LifeSciences
LifeNet Health
Global Sales Director
LifeSciences
LifeNet Health