Join Caroline Wuyts, MSc, for a deep dive into her research on pregnancy and monitoring glucose availability throughout the reproductive timeline of a mouse model. Pregnancy is a physiological challenge for the female body, and metabolic adaptations are necessary to maintain the balance between maternal and fetal growth. To gain insight into these pregnancy-induced adaptations, glucose was continuously monitored in mice via telemetry.
Highlights
- An introduction to gestational diabetes
- The benefits of continuous glucose monitoring (CGM) via telemetry
- A protocol for implantation of the transmitter body and glucose sensor in mice
- Changes in glucose levels and variability throughout pregnancy in mice
- A comparison of glycemia data in pregnant mice and humans
Webinar Summary
Caroline Wuyts begins this webinar with an introduction to gestational diabetes, its importance, and potential complications; importantly, the molecular mechanisms responsible are unknown, and therefore no specific treatments are currently available. When blood glucose following glucose administration has been studied in mice, this has traditionally been achieved using intermittent blood sampling, although animal models of gestational diabetes are needed to improve understanding.
“The standard techniques that exist to measure glycemia are very limited in resolution.”
In the series of experiments presented, a new method for continuous glucose monitoring (CGM) via telemetry in mice is introduced. The standard technique used for measuring the response to glucose is an oral glucose tolerance test (OGTT), in which glucose is administered orally, followed by repeated blood sample collection. The advantages of CGM, as opposed to intermittent blood sampling, are that animal handling is reduced, repeated blood draws are not required, and the extent of human intervention and influence is substantially reduced, collectively leading to decreased stress levels in the animal. CGM also provides considerably more data, enabling continuous 24-hour measurement, long-term monitoring, and detection of spontaneous glucose excursions.
“Telemetric implants are safe to be used in pregnant mice.”
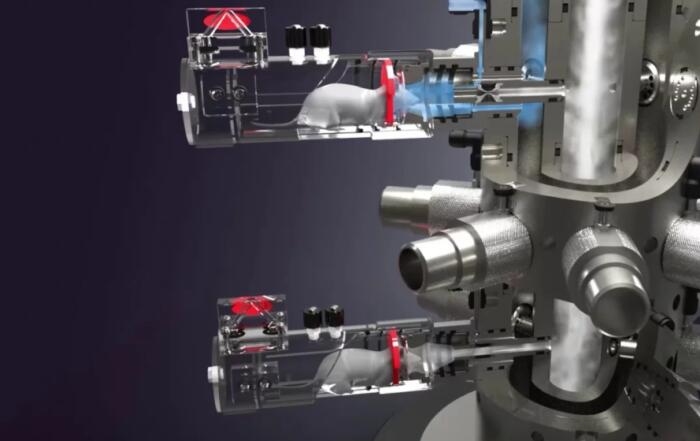
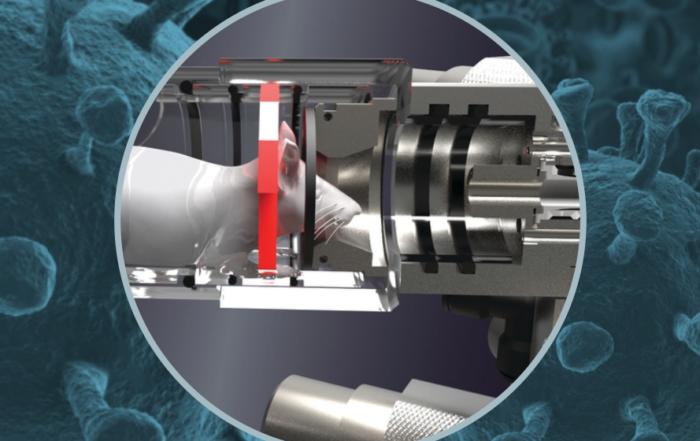
Caroline describes a protocol for the subcutaneous implantation of the transmitter body and glucose sensor for CGM, including important considerations and possible complications associated with the study of pregnancy in mice. Importantly, implantation does not interfere with movement, and mating can begin one week after surgery. Glucose values were monitored continuously from implantation until one week after pregnancy, at which time the transmitters were explanted to confirm their position. A series of four OGTTs were performed, beginning before pregnancy, at embryonic days 8 and 16, and at postpartum day 2. A sham group was also included to assess the influence of surgery and implantation on birth outcomes. No difference in body weight, litter size, or pup birth weight was observed, demonstrating that telemetric implants can be used safely in pregnant mice.
The results of these experiments are presented, and a strong correlation was observed between CGM and glucometer results following OGTT. In response to glucose administration, area under the blood glucose-time curve during pregnancy in mice was increased, suggesting pregnancy lowers glucose tolerance; this is also the case during human pregnancy. Interestingly, fasting glucose values were also increased during pregnancy in mice, whereas fasting glucose reportedly decreases during human pregnancy; the 6-hour fasting period commonly used in mice studies may not be equivalent to overnight fasting in humans.
“We can measure blood glucose every 10 seconds for the full pregnancy, and even before and after.”
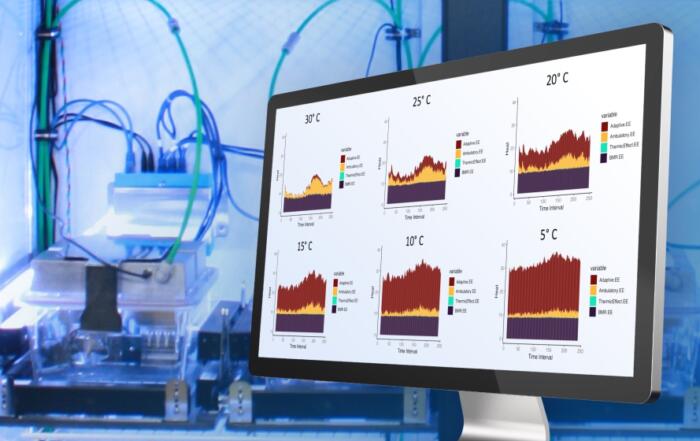
Due to the large quantity of data generated when using CGM, a Python script for automated data analysis was written. The outcome measures analyzed included standard transmitter parameters, glycemic variability, glucose excursions, animal activity (movement detected by changes in signal detection), and body temperature. As expected, greater animal activity was observed during the night than day, but decreased over the course of pregnancy; subcutaneous temperature also increased during pregnancy. Changes in glucose levels and variability throughout pregnancy are subsequently described in detail and compared with existing human data. Analysis of the CGM data identified numerous daily glucose excursions; these were compared with video recordings, revealing that the observed excursions coincided with eating. An objective method to define glucose excursions is then described, allowing for quantification of these episodes in the complete dataset. Effectively, the area under the blood glucose-time curve during excursions can be used as a proxy for OGTT results, but under normal conditions and without researcher intervention (as typically required for OGTT during oral glucose administration).
“Mice may be relevant models to study glycemic adaptations to pregnancy.”
The majority of glycemic adaptations are similar in mice and women, with the exception of fasting glucose, therefore mice may be a useful model of glycemic adaptations in pregnancy. CGM also enabled monitoring of glycemia during delivery for the first time and revealed decreasing glucose values at birth.
Ms. Wuyts concludes this webinar with a summary of protocols for safe and optimized implantation of telemetric implants in pregnant mice, CGM data together with video recording demonstrating that glycemic adaptations are similar in pregnant mice and pregnant women, and suggests that these models can be used to study gestational diabetes.
Resources
Q&A
- What diet are the mice in these experiments fed?
- Were there glucose changes or spikes when the animals were handled or experimenters entered the room they were housed in?
- For glucose tolerance tests, what food or substance was used?
- The area under the blood glucose-time curve increases after an OGTT during gestation, but glycemia increases and then decreases – how do you explain this?
- Have these experiments been done in mice that have had repeated pregnancies, and if so, how do the results differ from mice during their first pregnancy?
- Did transmitter implantation affect the outcomes of the pregnancy and did it influence mothering behavior?
- How long is the delay between food consumption and the associated glucose spike, and does it differ between pregnant and non-pregnant mice?
- Were there any issues with the implant collecting data due to delays between implantation and pregnancy?
- What is the time resolution for CGM?
- As CGM is becoming more popular in human pregnancy, what can we learn from animal models?
- Is the python script used for the data analysis open source or available?
- Where is this research headed in the future?
To retrieve a PDF copy of the presentation, click on the link below the slide player. From this page, click on the “Download” link to retrieve the file.
Presenters
PhD Candidate
Department of Cellular and Molecular Medicine
KU Leuven - VIB