Scientists discuss the value and utility of heart rate variability (HRV) as a preclinical tool for studying both cardiovascular, and non-cardiovascular related diseases in small and large animal models.
Heart rate variability (HRV) is a valuable tool used to investigate the balance between the sympathetic and parasympathetic branches of the autonomic nervous system. Parasympathetic nerves slow heart rate through the release of acetylcholine. Sympathetic nerves accelerate heart rate and force of contraction through the release of epinephrine and norepinephrine from nerve terminals and the adrenal glands. Heart rate and blood pressure spontaneously fluctuate even while resting or during steady-state conditions. HRV allows observation of the specific frequencies resulting from the fluctuations and provides insight to autonomic function. Thus, HRV is a valuable method that can be used to study various cardiovascular diseases including myocardial infarction, congestive heart failure, coronary artery disease, and hypertension, AND non-cardiovascular related diseases such as stroke, diabetes, alcoholism, cancer, and glaucoma, to name a few.
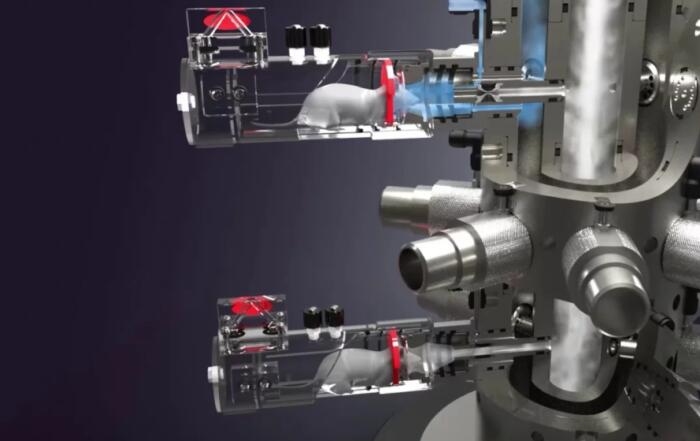
In this webinar, sponsored by Data Sciences International, scientists will present case studies investigating HRV using both rodent and large animal telemetry. First, Emma Karey will discuss how to distill autonomic function from rodent ECG telemetry recordings collected by the Chen lab in the Department of Pharmacology at the University of California, Davis. Specifically, she will discuss (1) how to identify and interpret the physiological significance of HRV in conscious, freely moving rodents, (2) how to efficiently obtain clean ECG recordings for downstream HRV analysis using select Data Insights software functions, and (3) how reductions in HRV can reflect cardiac dysfunction (in rodent models) that is caused—at least in some part—by changes in the cardiac vagal inputs to the SA node.
Following, John Wollard, Principle Research Technologist from the Lilach Lerman Renovascular Disease Research Laboratory at the Mayo Clinic, will present a case study involving a swine model of human disease related to renovascular dysfunction and metabolic syndrome. Specifically, he will share laboratory methodology, best-practices and preliminary data showing the value of heart rate variability as it relates to the investigation of metabolic dysfunction and hypercholesterolemia.
Resources
To retrieve a PDF copy of the presentation, click on the link below the slide player. From this page, click on the “Download” link to retrieve the file.
Presenters
Principal Research Technologist
Lilach Lerman Research Laboratory
Mayo Clinic
Postdoctoral Research Fellow
Environmental Medicine
NYU Grossman School of Medicine