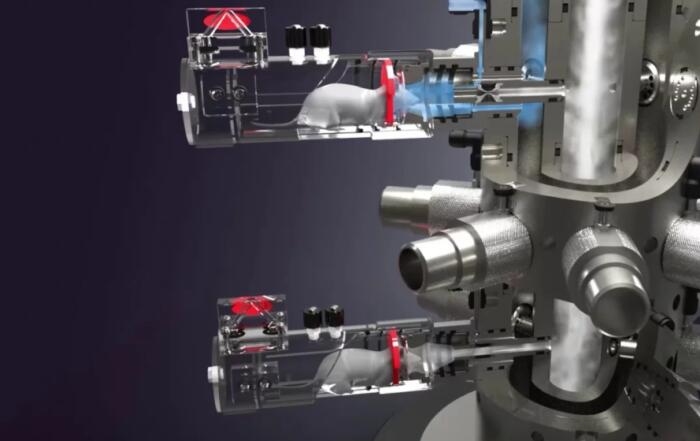
Dr. Aileen King presents data showing that even small alterations to experimental protocol and choice of model can substantially impact both welfare and data interpretation when studying blood glucose homeostasis in mice, using data from continuous glucose monitoring (CGM).
Highlights
- An introduction to CGM in mouse models
- A comparison of blood glucose levels and variability in male and female mice using CGM
- The effects of common experimental protocols on blood glucose levels and variability
- The benefits of refinement of protocols to measure blood glucose levels
Webinar Summary
Dr. King begins this webinar with an introduction to blood glucose homeostasis, the roles of insulin and glucagon on glucose uptake, storage, and production, and diabetes resulting from insulin deficiency leading to hyperglycemia. Animal models have been used in diabetes research for decades, but the assumption that the estrous cycle leads to greater variability in female mouse models has led to their underuse in preclinical diabetes research. In preclinical models, blood glucose measurement (for example during glucose tolerance tests, or GTTs) has commonly been achieved via repeated tail-prick sampling, requiring additional handling. CGM is an alternative method of glucose monitoring in mice, and the application of this method to mouse models is described. The benefits of CGM are discussed, including assessment of conscious animals in a normal state, minimizing human presence and intervention, reducing animal stress, and permitting collection of data not possible with tail prick sampling.
In the first series of experiments presented, normal blood glucose concentrations and fluctuations are reported and compared between male and female mice. Blood glucose concentrations were higher in male mice than females, with greater variability, independent of estrous cycle.
“. . . female mice have lower blood glucose concentration than males, female mice also have less variable blood glucose concentration . . . the estrous cycle does not lead to significant changes in blood glucose concentrations.”
In the next series of experiments, Dr. King describes changes in blood glucose concentration during common experimental procedures. For example, normal husbandry cage changes resulted in increased blood glucose for up to two hours following the change. Other simple procedures studied included welfare checks, handling, blood sampling, and intraperitoneal injection, all of which increased blood glucose levels in both males and females.
A standard GTT protocol is then described, beginning with fasting, a brief handling period, and glucometer measurement prior to glucose administration; the effects of these interventions on blood glucose levels assessed by CGM is presented.
“The increase in blood glucose concentrations seen during a GTT is partially due to the effects of the procedure itself.”
These results led to the question: does altering the GTT protocol affect animal welfare and experimental outcome? For example, how the food is removed, the duration of the fasting period, and the glucose administration route can all vary between different labs and experiments; the effects of these changes on blood glucose levels are presented. A series of changes to standard protocols are proposed, with the goal of improving animal welfare and improving data reliability and reproducibility; these include limiting fast duration to prevent hypoglycemia, avoiding whole cage changes, retaining bedding, and training mice to voluntarily ingest glucose gels. An additional advantage of voluntary oral glucose ingestion is that it is more physiologically accurate than glucose injection and does not require animal restraint. Importantly, although refinement of GTT methods reduced protocol-induced increases in blood glucose levels, the effects of drug treatment were still detectable.
“Refined protocols can be used to detect drug effects, despite blunted glucose responses in the control.”
Dr. King concludes this webinar with a summary of blood glucose concentrations and variability in male and female mice measured with CGM, the role of the estrous cycle on these measurements, and reiterated the proposed protocol changes.
Click to watch the webinar recording. To view the presentation full screen simply click the square icon located in the bottom-right corner of the video viewer.
Resources
Q&A
- Does CGM provide any data that cannot be obtained using conventional glucometers?
- Were the body weights of the mice taken into account?
- Did you study the effects of oral gavage on blood glucose levels?
- What were the ages of the mice and how might this affect the results?
- Are the observed differences in blood glucose between males and females due to insulin secretion or eating patterns?
- Were the mice handled prior to the studies presented?
- Would you expect similar results in rats?
- Would the stage of the estrous cycle have any effect on the results in diet-induced obesity studies?
- How are insulin secretion levels affected by fast duration?
- Would you expect similar results using refined protocols but without CGM?
- Does handling required for traditional GTTs have an effect on blood glucose levels?
To retrieve a PDF copy of the presentation, click on the link below the slide player. From this page, click on the “Download” link to retrieve the file.
Presenters
Senior Lecturer
School of Life Course Sciences
King's College London