Taurine: An Unexpected Anti-Aging Ally
Blog post by Jasmin Skinner
Due to the tremendous improvements of healthcare within the last 200 years, the average human lifespan has doubled since the early 1800s (1). Medical advances in testing, prognosis and treatment have made it possible for individuals to live well past a century, but not without significant health concerns that could limit their quality of life. There is rising interest in expanding an individual’s ‘healthspan’, which refers to “the period of life spent in good health, free from the chronic diseases and disabilities of aging” (2).
As of July 2023, over 16,000 publications containing the words “healthspan” appear on PubMed, with the number of publications growing exponentially in the last ten years. Many authors mention the idea of improving or increasing healthspan within humans and animal models, and while many studies explore potential ways of doing so, very few if any have demonstrated a reproducible, evolutionarily conserved way of increasing or improving healthspan; until recently.
Singh et al. present a novel study that describes and demonstrates how taurine, an amino acid widely used across the human body, has the ability to modulate lifespan and healthspan. Taurine is reported to be “one of the most abundant amino acids in the brain and spinal cord, leukocytes, heart and muscle cells, the retina and indeed almost every tissue throughout the body” (3). It is involved not only in the formation of bile salts, but also is a “broad-spectrum cytoprotective agent, … an organic osmolyte involved in cell volume regulation, … and plays a role in the modulation of intracellular free calcium concentration.”
Taurine has been proven to play a widely important role throughout the human body, but Singh et al. specifically highlight how an excess or deficiency of taurine could alter the health outcomes of middle-aged mice and assess whether taurine’s modulation is evolutionarily conserved in yeast and roundworms. This blog not only discusses the exciting findings from this paper, but also seeks to answer how a single amino acid could impact age-related health outcomes.
This blog post discusses how biological age can fluctuate in response to various physiological stressors, such as pregnancy and surgery. Read more about this study from Poganik et al., here.
How do you determine the effects of taurine supplementation?
The authors first determined whether taurine supplementation would be effective at improving health and lifespan in wild-type female mice and male C57BL/6J mice. To do so, taurine or a vehicle control was administered daily to the mice, beginning at 14 months and continuing until death. Mice were monitored at each dose administration for signs of ill health, such as refusal to eat/drink, dramatic weight loss or extreme lethargy. Additionally, a variety of cognition and muscle function tests were performed to determine the relative health of the mice compared to other mice their age (2).
In addition to monitoring the effects of taurine on mice, the authors sought to determine whether the effects observed in a murine model were evolutionarily conserved. To do so, they observed how supplemental taurine affects a species of roundworm, Caenorhabditis elegans, as well as in a few different yeast strains. The authors also administered supplemental taurine to Rhesus monkeys, Macaca mulatta. As previous studies have observed an increase in taurine following exercise, a human exercise cohort was utilized to determine how taurine and other metabolites change in response to exercise. Human clinical samples were also used to evaluate the decline in circulating taurine levels as people age and its association with adverse health outcomes like obesity and diabetes (2).
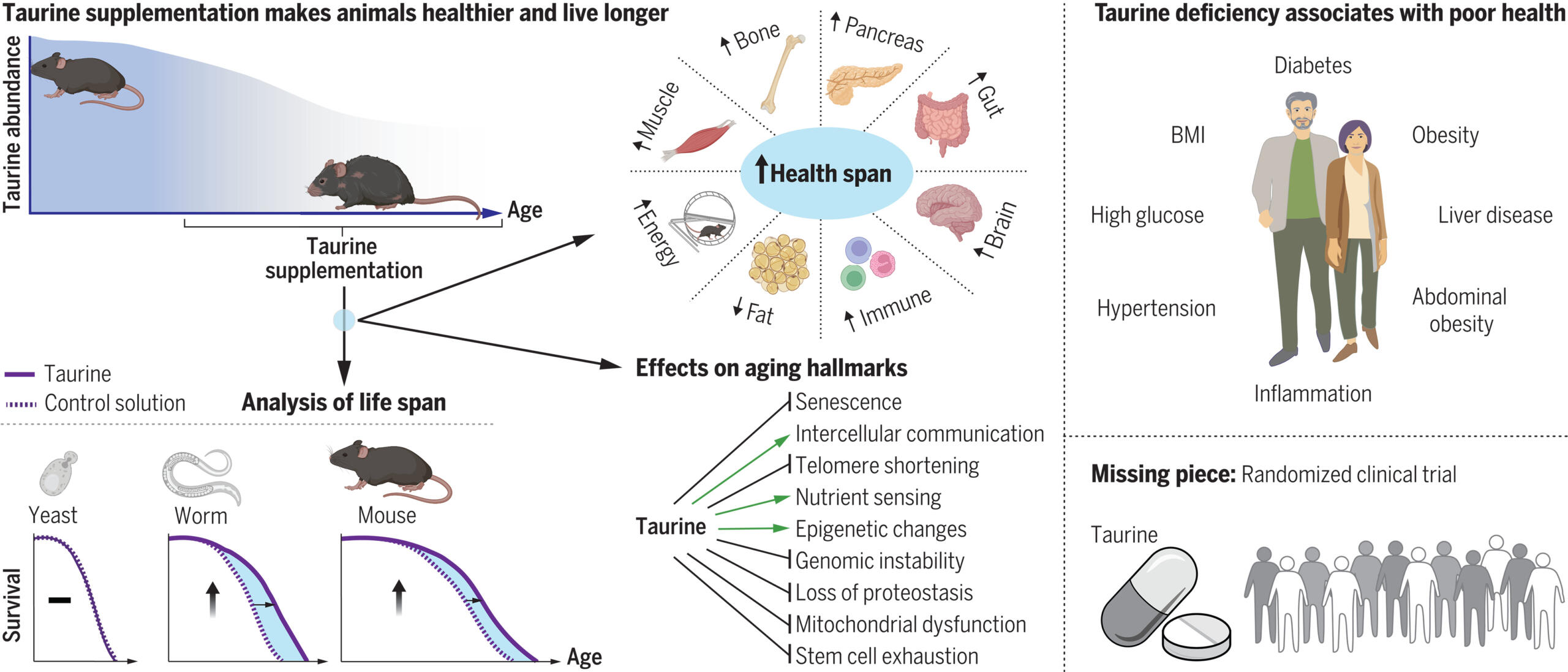
Figure 1. Figure 1: Study overview of how supplemental taurine can allow mice to live longer. As seen in the upper left, circulating taurine levels decrease with age, which, when supplemented to mitigate this decrease, results in a longer and healthier lifespan in both mice and roundworms. Additionally, taurine deficiency in humans is associated with a variety of health problems, as described in the top right. © 2023 Singh et al., licensed under CC BY 4.0.
What do taurine supplements do?
Based on human clinical sample data as well as results observed in mice and monkeys, taurine blood concentration decreases with age. Taurine supplementation was shown to increase the lifespan and life expectancy of mice, with taurine-supplemented mice living on average 10-12% longer than control mice. Additionally, mice that received taurine supplements had longer healthspans as well, with improved function within the immune system, bone, muscle, pancreas, brain and gut tissue, as well as a decrease in fat cells and an increase in physical activity. Taurine supplementation also increased the lifespan of the worms, but had no impact on the lifespan of unicellular yeast, indicating that perhaps a decrease in circulating taurine throughout one’s lifespan is unique to multicellular organisms. While the average lifespan of taurine-supplemented monkeys was not altered, their healthspan increased similarly to the mice, demonstrating an evolutionarily conserved connection between circulating taurine and overall health across a variety of species (2).
Taurine supplementation also mitigated several hallmarks of aging. Using various immunological and molecular assays, RNA analyses and muscle function tests, the authors found that it “reduced cellular senescence, protected against telomerase deficiency, suppressed mitochondrial dysfunction, decreased DNA damage, and attenuated inflammation” (3). The wide distribution of taurine in “almost every tissue throughout the body”, along with the links between taurine deficiency and various diseases like severe cardiomyopathy and renal dysfunction, further highlights the significant effects of supplemental taurine. The observed increase in taurine concentrations following exercise is also likely to contribute to its ability to increase health- and lifespan (2).
Have other studies confirmed these findings?
While this study is the most recent example of how supplemental taurine can improve one’s health, several previous studies have observed this effect in other areas of the body. Taurine supplementation has been observed to improve functional recovery in animal models after traumatic brain injury or ischemic stroke, likely due to its role as an inhibitory transmitter (3). It has also shown promise in a wide variety of animal model disease states, such as preventing cell death in the hippocampus following kainite-induced epilepsy (3). A recent study from Liu et al. report that in a mouse model of subarachnoid hemorrhage, in which bleeding occurs in the space between the brain and the skull, taurine was able to “reduce early brain injury and ferroptosis by Akt/GSK3β/β-catenin signaling pathway, a pathway reported to be involved in promoting glioma, a kind of brain cancer, and neural stem cell proliferation” (4,5,6,7). While the many applications of supplemental taurine have shown tremendous promise in animal models, it is important to note that taurine is also one of the most prevalent metabolites within the central nervous system and can vary in concentration between various brain regions and developmental stages, as well as between species (7). The true extent of taurine’s effects on the nervous system will require further characterization to unravel the unique mechanisms that underpin this mysterious metabolite.
Future implications: how can taurine be used practically?
Despite the additional research required to validate these findings in humans, the currently available research demonstrates its wide potential across multiple disciplines. While no outstandingly negative effects of taurine supplementation have been reported thus far, it is likely too early to stock up on taurine supplements. Randomized, long-term clinical trials will be necessary to assess how taurine supplementation affects human health and longevity (2).
About the Author
About the Author

Jasmin Skinner is an undergraduate student at the University of Western Ontario completing a Specialization in Biology and a Minor in Chemistry, with focused interest in applying these concepts to environmental conservation. As a lover of the outdoors and the arts, much of her time is spent in nature and within the local London art community, creating and connecting with all walks of life. After graduating, she hopes to continue her passion of finding unconventional solutions to environmental issues by working with nature, not against it.
References
- Ruggeri A. Do we really live longer than our ancestors? BBC Future. October 2018. Accessed July 2023. https://www.bbc.com/future/article/20181002-how-long-did-ancient-people-live-life-span-versus-longevity
- Singh P, Gollapalli K, Mangiola S, et al. Taurine deficiency as a driver of aging. Science. 2023;380(6449). doi: 10.1126/science.abn9257
- Rafiee Z, García-Serrano AM, Duarte JMN. Taurine supplementation as a neuroprotective strategy upon brain dysfunction in metabolic syndrome and diabetes. Nutrients. 2022;14(6):1292. doi: 10.3390/nu14061292
- The Johns Hopkins University, The Johns Hopkins Hospital, and Johns Hopkins Health System. Subarachnoid Hemorrhage. John Hopkins Medicine. Accessed July 2023. https://www.hopkinsmedicine.org/health/conditions-and-diseases/subarachnoid-hemorrhage#:~:text=A%20 subarachnoid%20 hemorrhage%20 means%20that,increasing%20pressure%20on%20the%20brain
- Liu C, He P, Guo Y, et al. Taurine attenuates neuronal ferroptosis by regulating GABAB/AKT/GSK3β/β-catenin pathway after subarachnoid hemorrhage. Free Radic Biol Med. 2022;193(2):795-807. doi: 10.1016/j.freeradbiomed.2022.11.003
- Tang Z, Yang G, Wang X. AKT/GSK-3β/β-catenin signaling pathway participates in erythropoietin-promoted glioma proliferation. J Neurooncol. 2020;149(2):231-242. doi: 10.1007/s11060-020-03602-9
- Liao Z, Zhou X, Li Siyu, et al. Activation of the AKT/GSK-3β/β-catenin pathway via photobiomodulation therapy promotes neural stem cell proliferation in neonatal rat models of hypoxic-ischemic brain damage. Ann Transl Med. 2022;10(2):55. doi: 10.21037/atm-21-5619