The Sweet Truth: Unwrapping the Science of Semaglutide
Blog post by Jasmin Skinner
The question of whether obesity is a disease has been the subject of debate within the medical community since it was defined. In 1998, the National Institutes of Health (NIH) was among one the first major institutions to categorize obesity as a disease, setting a precedent that would spark widespread discussion, but not necessarily widespread agreement (1). The American Medical Association’s (AMA) Committee on Science and Public Health presented a counter-argument in 2012, contending that obesity did not meet the medical criteria for a disease state. The panel stated that there was insufficient data to describe obesity as a disease and that it does not fit the medical definition of a disease state, as “it has no symptoms, and it’s not always harmful” (2). Despite these counter-arguments, the AMA officially recognized obesity as a “disease state requiring treatment and prevention efforts” in 2013, a decision that was somewhat unexpected given the stance of their Committee.
With current estimates stating 38% of the world’s population now meet the criteria for being overweight or obese, the question of how best to address obesity becomes ever more pressing. Regardless of whether it is officially classified as a disease, it is clear that obesity is a significant public health issue that requires increasing attention and efforts towards prevention and treatment.
Controversy over how obesity should be managed drives further debate among experts, with most agreeing that the best long-term solution is to alter one’s eating and exercise habits. However if this proves ineffective or unfeasible for patients, medical intervention is often required. There are a wide array of options to treat obesity, each with their own varying long-term effectiveness and adverse effects. Some examples include intermittent fasting, restricted caloric diets, electrical stimulation to the abdomen with an implanted device, and bariatric surgery, which surgically adjusts an individual’s digestive system (3).
While no weight loss solution will be perfect for everyone, a recent and emerging weight loss medication has been advertised and discussed just about everywhere. From billboard ads to TikTok hashtags, Semaglutide, also known as Ozempic and Wegovy, is the weight loss solution everyone seems to be talking about. In this blog, we join the conversation about this controversial weight loss medication, discussing its mechanism of action, intended and off-label use, recent rise to popularity, and currently known long-term effects.
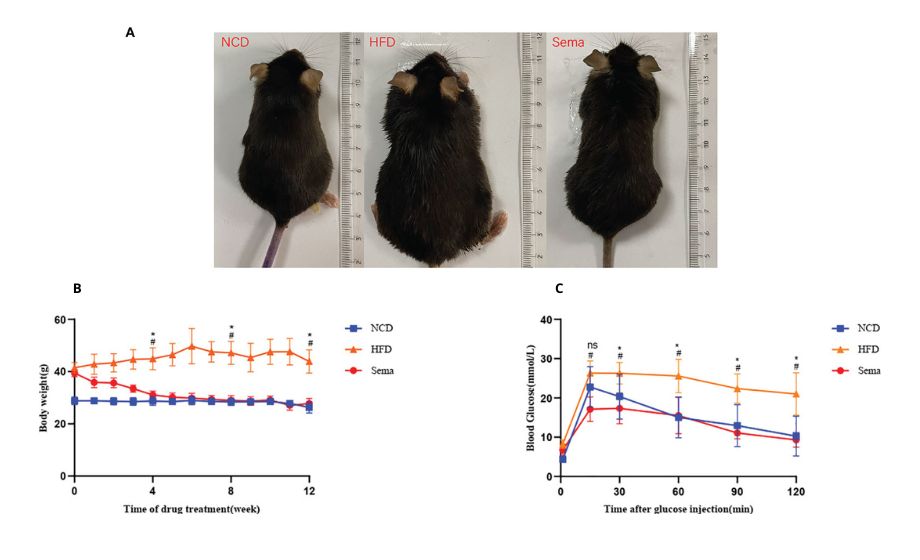
Figure 1: Effects of Ozempic, a.k.a semaglutide (Sema) compared to a normal control diet (NCD), and a high fat diet (HFD) after 12 weeks. This figure demonstrates how visually effective semaglutide is (a), as well as how the body weight of the three test groups changed over the 12 weeks (b). Finally, (c) demonstrates how blood glucose fluctuates after a glucose injection. © 2022 Yue et al., licensed under CC BY 4.0.
How does semaglutide work for weight loss?
Ozempic and Wegovy are branded names for the same drug, known as semaglutide. This drug is meant to regulate blood sugar levels for patients with type 2 diabetes, and is often used after other medications fail to adequately control blood sugar levels. Semaglutide is a glucagon-like peptide-1 (GLP-1) receptor agonist (RA). This means that it replicates the structure and function of glucagon-like peptide 1, binding to the GLP-1 receptor and stimulates insulin secretion after oral glucose consumption through the incretin effect (4). Normally, when a meal is consumed, GLP-1 and glucose-dependent insulinotropic polypeptides (GIPs) are secreted from the intestines to the bloodstream, where they then travel to the pancreas and bind to beta cells which produce insulin (4). For patients with type 2 diabetes, this process can be blunted or even absent, but through the use of a GLP-1 RA like semaglutide, patients can revive their body’s insulin excretion ability – with great effectiveness.
A thorough discussion and timeline of obesity treatments that are currently available, their limitations, why animal models are so crucial to this field of study, and some up-and-coming anti-obesity pharmacological treatments. READ HERE
A clinical study showed how type 2 diabetics already taking two other antidiabetic drugs that intensified this with a GLP-1 RA were “more likely to reach their blood sugar goals and lose weight compared to adding a further oral antidiabetic drug(s) or insulin.” (5) This mention of weight loss is important to note, as this is what semaglutide is most known for. As obese people are 80 times more likely to develop type 2 diabetes, weight loss is often a necessary part of diabetic treatment and management (6). How one drug can have such a profound effect on patients experiencing both obesity and type 2 diabetes is rather interesting, but is better explained by examining where GLP-1 receptors are found within the brain.
GLP-1 receptors are found on the lateral hypothalamic neurons, a region of the brain associated with controlling feeding, blood pressure, heart rate, water intake and sodium excretion (7). Additionally, the lateral hypothalamus is a key reward-control locus in the brain, and studies have found in a rodent model that selective activation of GLP1 receptors in the lateral hypothalamus resulted in a decrease in food motivation, food intake, and body weight (8). Additionally, a pharmacological blockade of GLP-1 receptors within the lateral hypothalamus resulted in an increase in food motivation in male rats, but not female rats.
To put into perspective just how effective semaglutide can be for weight loss, a study published in June 2023 found that participants lost around 5% of their body weight (approximately 10 pounds on average) in 30 weeks. The subjects also experienced a reduction of waist size of about 1.6 inches in that same time period (9). This rapid rate of weight loss is further validated by a 2022 review of semaglutide funded by Novo Nordisk stating that participants lost, on average, 14.9%-17.4% of their body weight after 68 weeks (10). The impressive success rates of weight loss, backed by reviews like the 2022 study of semaglutide, have not only showcased Ozempic’s effectiveness but also raised intriguing questions about its surging off-label popularity.
How has semaglutide become so popular?
Since the FDA approval of semaglutide-based drugs like Ozempic in 2017 and Wegovy in 2021, consumer interest has been steadily sky-rocketing (11, 12). This growing customer curiosity can be attributed to a variety of factors, but was likely initiated by the immense ‘marketing’ budget set forth by Novo Nordisk, the manufacturer of both Ozempic and Wegovy. In 2022, Novo Nordisk spent around 9 million dollars purchasing more than 457,000 meals for prescribing doctors (13). While marketing to physicians is likely not the only reason for semaglutide’s success, having thousands of doctors fully versed in the specifics of this drug has likely spurred its popularity.
Specific requests from patients are also likely a major factor, as studies have found that patients requesting a particular medication are more likely to receive it than if no request were made (14). As an extreme example, a doctor licensed in Nova Scotia but practicing in Texas was recently suspended as he issued over 13,000 prescriptions for semaglutide in January and February 2023 alone (15). This immense influx of prescriptions is likely not just due to industry pressure and potential benefits, but also from patients specifically requesting the drug as it permeates their daily lives.
With approximately 4000 active ad campaigns on Facebook and Instagram alone, semaglutide is getting harder and harder to ignore. As a comparison, viagra, another heavily marketed drug, has approximately 800 active Instagram ads, and 950 active Facebook ads (16). Additionally, discussion of the benefit of Ozempic on apps like TikTok have also contributed to its popularity. At the time of writing, the hashtag #Ozempic has around 1.2 billion views in total on TikTok, and, while all of those may not be direct users of the drug, this number highlights just how much public interest semaglutide and its brand names have garnered (17). All this discussion online is furthered by traditional marketing campaigns, with many billboards, print ads, and other marketing avenues pushing this drug to the forefront of many individual’s minds. This drug could not have become so popular without it truly being effective at promoting weight loss, but are these effects sustainable in the long-term?
What are the long-term effects of Ozempic, Wegovy, and other similar drugs?
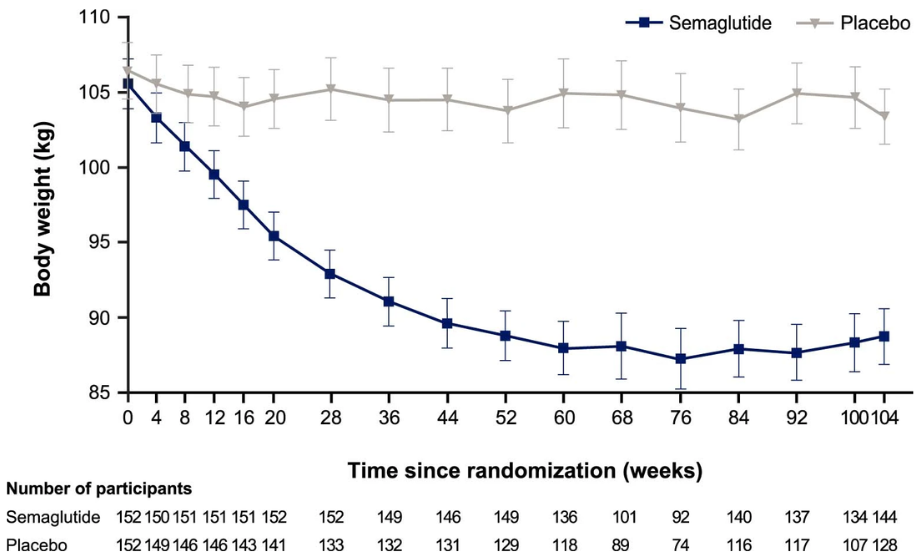
Figure 3: Mean body weight (kg) of participants taking semaglutide or a placebo over a two year observational trial. © 2022 Garvey et al., licensed under CC BY 4.0.
As semaglutide has only been available since 2017, long-term clinical data are not yet available to assess its impact on an individual’s health in the future, or if this decreased body weight can be maintained. After two years on these kinds of medications, Garvey et al. reported a 15.2% reduction in weight compared to the placebo group of 2.6%. To put that into perspective, if a 300 pound person were to lose 15% of their body weight, they’d be losing 45 pounds; whereas if they lost 2% of their weight, they’d lose 6 pounds and weigh nearly the same (18). While two years on a medication is relatively insignificant in the grand scheme of life, it does demonstrate that prolonged usage of this drug sustains its weight-loss effects over a two-year time period.
One repeatedly observed side effect of this drug is mild-to-moderate gastrointestinal adverse events, which Garvey et al. reported to affect 82.2% of patients who received semaglutide, compared to 53.9% of the placebo group. The most frequently encountered adverse effects included nausea, diarrhea, vomiting, and constipation. However, these gastrointestinal gripes are likely worth the trouble when compared to the positives of semaglutide-based drugs, as they were reported to have “improved a range of cardiometabolic risk parameters, including waist circumference, systolic and diastolic blood pressure, HbA1c levels, total cholesterol, low-density lipoprotein cholesterol, very-low-density lipoprotein cholesterol and triglycerides” (18).
Is Ozempic the solution to obesity?
It’s clear that Ozempic and Wegovy are effective for weight loss and for managing blood sugar in type 2 diabetics. However, longitudinal studies are ultimately required to determine whether these drugs will be effective at maintaining a healthy weight. At present, semaglutide appears to be the most promising option for weight loss among available medications, but further research is required to provide a more comprehensive picture of its efficacy and safety profile.
About the Author
About the Author

Jasmin Skinner is an undergraduate student at the University of Western Ontario completing a Specialization in Biology and a Minor in Chemistry, with focused interest in applying these concepts to environmental conservation. As a lover of the outdoors and the arts, much of her time is spent in nature and within the local London art community, creating and connecting with all walks of life. After graduating, she hopes to continue her passion of finding unconventional solutions to environmental issues by working with nature, not against it.
References
- Rosen H. Is obesity a disease or a behavior abnormality? Did the AMA get it right? Mo Med. Mar-Apr 2014;111(2):104-8. Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6179496/
- Brown H. How obesity became a disease. The Atlantic. Mar 2015. Accessed Aug 2023. https://www.theatlantic.com/health/archive/2015/03/how-obesity-became-a-disease/388300/
- U.S. Department of Health and Human Services. Treatment for overweight & obesity. National Institute of Diabetes and Digestive and Kidney Diseases. Accessed Aug 2023. https://www.niddk.nih.gov/health-information/weight-management/adult-overweight-obesity/treatment
- Mahapatra MK, Karuppasamy M, Sahoo BM. Semaglutide, a glucagon like peptide-1 receptor agonist with cardiovascular benefits for management of type 2 diabetes. Rev Endocr Metab Disord. Jan 2022;23(3):521-539. doi: 10.1007/s11154-021-09699-1
- Novo Nordisk. Switching to Ozempic® from another GLP-1 RA significantly reduced blood sugar and weight in people with type 2 diabetes in routine clinical practice. PharmiWeb.com. Jun 2020. Accessed Aug 2023. https://www.pharmiweb.com/press-release/2020-06-17/switching-to-ozempic-from-another-glp-1-ra-significantly-reduced-blood-sugar-and-weight-in-people-with-type-2-diabetes-in-routine-clinical-practice
- Diabetes.co.uk. Diabetes and obesity. Diabetes.co.uk. September 2022. Accessed Aug 2023. https://www.diabetes.co.uk/diabetes-and-obesity.html#:~:text=In%20fact%2C%20obesity%20is%20believed,BMI%20of%20less%20than%2022.
- Fakhoury M, Salman I, Najjar W, Merhej G, Lawand N. The lateral hypothalamus: an uncharted territory for processing peripheral neurogenic inflammation. Front Neurosci. Feb 2020;14:101. doi: 10.3389/fnins.2020.00101
- López-Ferreras L, Richard JE, Noble EE, et al. Lateral hypothalamic GLP-1 receptors are critical for the control of food reinforcement, ingestive behavior and body weight. Mol Psychiatry. Sep 2017;23:1157-68. doi: 10.1038/mp.2017.187
- Wojtara M, Syeda Y, Mozgala N, Mazumder A. Examining off-label prescribing of ozempic for weight-loss. Qeios. June 2023;T6Y97S. doi: 10.32388/T6Y97S
- Bergmann NC, Davies MJ, Lingvay I, Knop FK. Semaglutide for the treatment of overweight and obesity: a review. Diabetes Obes Metab. May 2022; 25:18-35. doi: 10.1111/dom.14863
- Drugs.com. Ozempic FDA approval history. Drugs.com. Accessed Aug 2023. https://www.drugs.com/history/ozempic.html
- U.S. Food & Drug Administration. FDA approves new drug treatment for chronic weight management, first since 2014. FDA.gov. Jun 2021. Accessed Aug 2023. https://www.fda.gov/news-events/press-announcements/fda-approves-new-drug-treatment-chronic-weight-management-first-2014
- Prater E. Ozempic manufacturer Novo Nordisk spent $11 million last year ‘wining and dining’ doctors. Experts slam the move as a breach of doctor-patient trust. Fortune Well. Jul 2023. Accessed Aug 2023. https://fortune.com/well/2023/07/21/ozempic-novo-nordisk-meals-travel-prescribing-doctors/
- McKinlay JB, Trachtenberg F, Marceau LD, et al. Effects of patient medication requests on physician prescribing behavior: results of a factorial experiment. Med Care. Apr 2014;52(4):294-9. doi: 10.1097/MLR.0000000000000096
- Patil A. Doctor tied to thousands of Ozempic prescriptions in B.C. has N.S. licence suspended. CBC News. Apr 2023. Accessed Aug 2023. https://www.cbc.ca/news/canada/nova-scotia/nova-scotia-ozempic-doctor-1.6804461
- Ireland N. Rise of Ozempic ads in Canada raising concern. What are the risks? The Canadian Press. Jun 2023. Accessed Aug 2023. https://globalnews.ca/news/9779972/ozempic-ads-canada-concerns/
- TikTok. “Ozempic”. Accessed Aug 2023. https://www.tiktok.com/tag/ozempic?lang=en
- Garvey WT, Batterham RL, Bhatta M, et al. Two-year effects of semaglutide in adults with overweight or obesity: the STEP 5 trial. Nat Med. Oct 2022;28:2083-91. doi: 10.1038/s41591-022-02026-4