Recent Advances in Anti-Obesity Pharmacological Research
Scientific article by Nina Culum, MSc
The global prevalence of obesity has nearly tripled since 1975 and, based on current trajectories, more than half of American adults are projected to be obese by 2030 [1, 2]. Left untreated, individuals with obesity are at an increased risk of developing many serious diseases and health conditions including hypertension, type 2 diabetes (T2D), cardiovascular disease (CVD), and musculoskeletal disorders [3]. Depending on its duration, obesity is also associated with a 5-20 year decrease in life expectancy [1]. In addition to these health consequences, obesity poses a great financial burden; more than $190 billion is spent annually in the United States to treat obesity-related diseases [4].
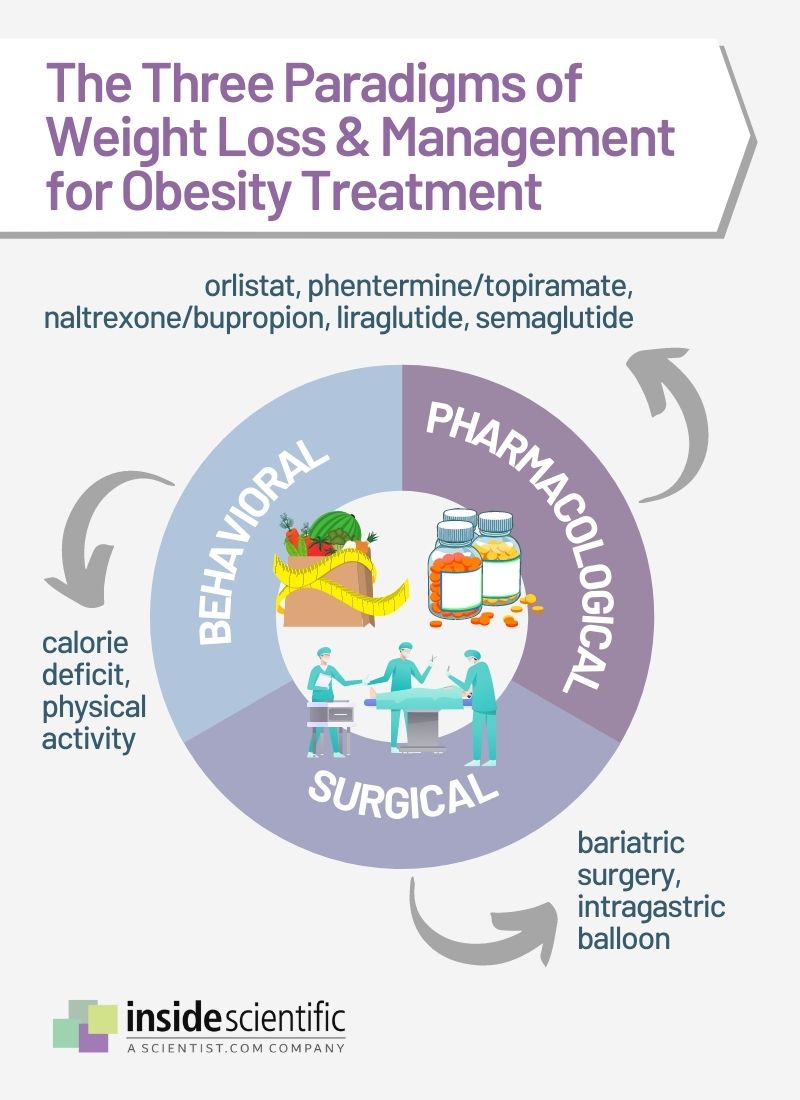
The fundamental cause of obesity is an energy imbalance between calories consumed and expended. Obesity is increasingly being recognized as a chronic, degenerative disease rather than just a risk factor for other noncommunicable diseases, which serves to destigmatize obesity and counter the belief that it results from insufficient self-discipline or “laziness” [4]. Research has suggested that there is a distinct pathophysiology that leads to excess fat accumulation as well as homeostatic mechanisms that challenge weight loss and exacerbate weight gain [4]. If preventative measures (e.g., diet and physical activity) fail or are not accessible, individuals can seek treatment for obesity in the forms of behavioral, pharmacological, and/or surgical interventions (Figure 1).
Current Obesity Treatments and Their Limitations
Behavioral modifications for a net calorie deficit can be adopted to achieve weight loss, through increased physical activity, decreased caloric intake, or a combination of both. However, many individuals experience a plateau in weight loss in the long term or a rebound in weight due to metabolic adaptations or poor adherence [5]. Surgery is currently the most effective method for weight loss and can lead to great improvements in obesity-related diseases. Despite its efficacy, the associated risks do not always outweigh the benefits; weight-loss surgeries can lead to gastroesophageal reflux, anastomotic leaks, internal bowel herniation and obstruction, and malnutrition [5].
Pharmacological treatment is recommended in addition to calorie restriction and physical exercise for weight loss maintenance, but effective options are limited and unpleasant gastrointestinal side effects are numerous. The first anti-obesity medication that could achieve greater than 10% body weight reduction was approved in 2021; semaglutide, a glucagon-like peptide-1 (GLP1) receptor agonist formulated as an injection, has demonstrated unprecedented weight loss results in clinical studies [6, 7]. Although it is generally well-tolerated, adverse gastrointestinal effects are still prevalent, and it remains unknown if an oral formulation will achieve comparable weight reduction results [7].
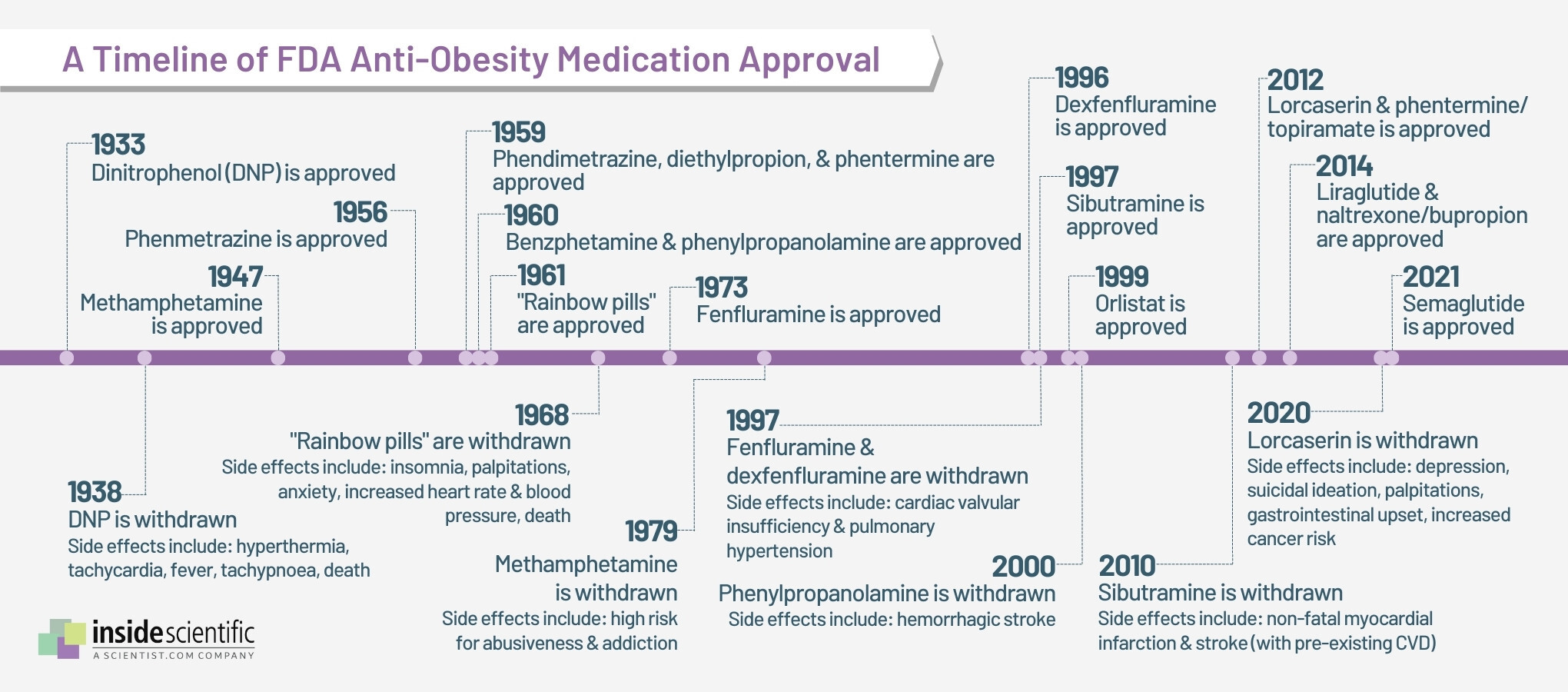
The lack of variety in effective anti-obesity medications is largely due to the fact that the molecular mechanisms controlling appetite were poorly understood until recent years [4]. Historically, many anti-obesity medications have been withdrawn from the market due to adverse cardiovascular effects, increased suicide risk, and drug abuse (Figure 2). An ideal anti-obesity drug should not only significantly and sustainably improve weight and obesity comorbidities, but also lack the potential for abuse and other adverse effects [4].
Figure 2: A rocky timeline of anti-obesity medication approval in the United States. Created with data from ref. 4.
The Need for Animal Models
The urgent need for safer and more potent anti-obesity medications has necessitated animal models to discover, validate, and optimize novel therapeutics for safe use in humans [2]. Large animal models, especially non-human primates (NHPs), are particularly translationally relevant to humans, but they are expensive to maintain, have long life cycles, and often raise important ethical considerations [2]. Rodent models of obesity and T2D are the most common and numerous, as many technologies are available for genetic manipulation and metabolic phenotyping, and they have short reproductive cycles. Mouse models in particular have been at the forefront of obesity research advances in the past 20 years, with over half of all preclinical animal research done in mice [2].
Diet-induced obesity (DIO) models are commonly used as they mimic obesity development in humans better than genetic models; typically, rodents are given calorie-dense foods that are highly enriched in fats or sugar [8]. Obesity is not as well-defined in experimental animals as it is in humans, but rather considered to be any significant increase in marker levels (e.g., body composition, condition index, total body weight gain, adipose tissue depot weight, etc.) relative to control animals [8]. Although the DIO model replicates elements of human obesity pathogenesis, many factors such as mouse strain, sex, age, and diet can influence experimental outcomes, and should therefore be considered when interpreting results and assessing translational values [2].
Mouse Metabolic Measurements
Mouse metabolic activity can be measured by direct or indirect calorimetry, although the former has fallen out of popularity since it is technically challenging [9]. Direct calorimetry involves measuring heat produced by the animal, but assumes that no heat is stored in the animals’ body while it is being measured, and can thus introduce a substantial amount of error [9]. Alternatively, indirect calorimetry or respirometry can estimate the amount of heat produced by the mouse by measuring the components of the metabolic process that generate heat [9, 10]. Typically, mice are placed in containers through which air is flowed at a known rate, and the amount of oxygen produced and carbon dioxide consumed is measured, which can provide insights into substrate oxidation [9]. Indirect calorimetry is used in almost all animal metabolic measurements conducted today [10].
How has Columbus Instruments advanced preclinical obesity research?
Columbus Instruments has pioneered the metabolic phenotyping systems that are commonly used for studying animal models of obesity. The Comprehensive Lab Animal Monitoring System (Oxymax-CLAMS) employs indirect calorimetry to monitor energy expenditure in real time, while also providing the infrastructure for physical activity, core body temperature, and food and water intake measurements (Figure 3) [11]. In addition to monitoring energy balance over long time periods, Oxymax-CLAMS can be combined with exercise treadmill regimens to measure the impact of exercise on energy balance [12], gene expression [13], and maternal health and offspring development [14].
Exercise is known to increase energy expenditure and can be induced in rodents through forced treadmill or voluntary wheel running, the latter of which is less stressful for the animal, allows for more natural running behavior, and is often implemented in both cage living spaces and metabolic phenotyping systems [15]. Voluntary running wheels have also been used to study how physical activity affects adipose tissue function, while other solutions are in development [16]. Although wheels and treadmills are often used to study exercise and endurance, little is known about the potential effects of voluntary resistance exercise. The new “ergo metric running wheel” by Columbus Instruments enables a mouse’s natural instinct to run while applying precise resistance to the wheel (Figure 4). Through computer control, resistance can be held constant or increased to simulate uphill running. Though still in its infancy, the results of these experiments are highly anticipated.

Figure 3: Mouse in a Columbus Instruments Oxymax-CLAMS-HC metabolic cage.

Figure 4: The new Columbus Instruments “ergo metric running wheel” with programmable resistance.
Accurately measuring mouse metabolism is crucial for understanding the effects of pharmacological treatments on energy metabolism [9]. Modern metabolic phenotyping systems have allowed for facile and cost-effective analyses of weight gain mechanisms, which is required for effective anti-obesity pharmacotherapy development [17, 18].
Emerging Anti-Obesity Pharmacological Treatments
Although the weight loss effects of pharmacotherapy generally translate well from rodents to humans, maximal efficacy is usually two to four times lower in humans than it is in rodents, possibly due to higher mass-specific energy expenditure in rodents than humans and a greater contribution of brown adipose tissue (BAT) to metabolic rate [4]. Furthermore, many anti-obesity therapies fail in clinical trials because rodents are resistant to adverse cardiovascular and pulmonary drug effects, and preclinical models often also fail to account for the heterogeneity of patients with obesity [4]. Despite the numerous challenges in the field, several treatments remain promising, including incretin-based therapies, mitochondrial uncouplers, growth differentiation factor 15 (GDF15), and peptide tyrosine tyrosine (PYY), as well as many different types of phytochemicals.
Incretin-Based Therapies
The emergence of peptide analogues in incretin-based therapies has lessened the frequency of adverse gastrointestinal side effects and has enabled more sustained and effective anti-obesity treatment [4]. A 2019 study reported that peptide-based glucose-dependent insulinotropic polypeptide (GIP) receptor agonists optimized for selectivity, cross-species activity, and action duration could consistently lower body weight in DIO mice [19]. Since mammalian organisms govern energy balance through more than one hormone, a major breakthrough in anti-obesity research has been the discovery of poly-agonists that simultaneously target GLP1, GIP, and/or glucagon receptors [4]. Unimolecular GLP1/GIP co-agonism in particular has been shown to correct obesity and glucose tolerance in both obese rodents and NHPs [20]. Tri-agonism, first reported in 2015, has also achieved superior body weight and plasma cholesterol reductions in DIO mice, and some have recently advanced to clinical trials [21].
Mitochondrial Uncouplers
Mitochondrial uncouplers work by decreasing mitochondrial coupler efficiency, which can have beneficial health effects. However, mitochondrial uncouplers are generally cytotoxic at high concentrations due to reduced ATP concentration as well as plasma and lysosomal membrane depolarization and permeabilization [4]. The uncoupler 2,4-dinitrophenol (DNP) initially yielded promising anti-obesity results, but was withdrawn from the market and banned from therapeutic use due to multiple adverse effects, including death in some cases. At non-toxic doses, mitochondrial uncouplers can protect cells against death, and so they are still attractive targets for anti-obesity medications. An oral controlled-release DNP formulation recently achieved low-level hepatic mitochondrial uncoupling in rats that reversed hypertriglyceridemia, insulin resistance, hepatic steatosis, and diabetes, while not being associated with any systemic toxicity [22].
N5,N6-Bis(2-fluorophenyl)[2,1,3]oxadiazolo[4,5-b]pyrazine-5,6-diamine (BAM15) is a novel mitochondria-specific protonophore uncoupler that possesses similar potency to DNP in increasing energy expenditure, but without serious adverse effects. Recently, BAM15 has been demonstrated to reverse DIO and insulin resistance in mice; oral administration of BAM15 can increase nutrient oxidation and reduce body fat mass without altering food intake, lean body mass, or body temperature [23]. Another study demonstrated that BAM15 could stimulate energy expenditure as well as glucose and lipid metabolism to protect against DIO [24]. BAM15-treated mice in this study were resistant to weight gain and exhibited improved body composition compared to control mice as well as glycemic control independent of weight loss.
GDF15 and PPY-Based Therapies
GDF15 is physiologically expressed at low concentrations in multiple tissues, but its levels increase where there is tissue injury, cancer, metabolic disease, CVD, or inflammation [4]. A recent study proposed GDF15 as a potential pharmacotherapy for obesity treatment; recombinant GDF15 was shown to induce weight loss in DIO mice and NHP [25]. However, its sustained efficacy has not yet been demonstrated and safety risks have not been assessed. The major active form of PPY is a high-affinity agonist for the neuropeptide Y receptor type 2; in 2021, it was shown in both rodents and humans that this active form decreases food intake and body weight [26]. Long-acting PPY analogues for obesity treatment are therefore in development, with some already in clinical trials.
Polyphenols
Research into phytochemicals for pharmacological obesity management has also gained interest in recent years as several plant-derived chemical constituents have demonstrated significant anti-adipogenic and anti-obesogenic effects [27]. Many polyphenols, for example, have been shown to prevent obesity development through several mechanisms, including increased lipolysis and fatty acid β-oxidation, decreased lipogenesis, adipose tissue and insulin sensitivity modulation, inflammatory response and oxidative stress reduction, and energy expenditure stimulation [28].
Polyphenols like glabridin, ferulic acid, resveratrol, and quercetin have demonstrated anti-obesity effects in mouse models. A glabridin analogue has recently demonstrated dose-dependent effects on adiposity and fatty liver in HFD-fed obese mice, primarily through increased energy expenditure [39]. Administration of a phenolic-rich extract containing ferulic acid and anthocyanins in HFD-fed obese mice also led to reduced adipose fat mass and adipocyte size, as well as decreased glucose, insulin, and triglyceride levels [30]. Resveratrol has recently been shown to mitigate body weight gain without influencing daily energy intake in HFD-fed obese mice while improving glucose tolerance and insulin sensitivity [31]. Quercetin, one of the most abundant flavonoids found in human dietary sources, has been reported to significantly reduce adipocyte size, attenuate adipose inflammation, and prevent systemic insulin resistance in HFD-fed rats [32].
Alkaloids
The alkaloid capsiate, a capsaicin analog, has been shown to enhance energy expenditure comparably to capsaicin without unpleasant side effects like gastritis; in one study, capsiate intake in HFD-fed mice combined with exercise training reduced the abdominal fat rate compared to exercise training alone [33]. Alkaloid combination with polyphenols has also exhibited anti-obesity effects in mice; chlorogenic acid and caffeine administration in HFD-fed obese mice effectively decreased body weight gain and intraperitoneal adipose tissue weight [34].
Terpenoids
The carotenoid lycopene has been shown to exhibit anti-oxidative, anti-inflammatory, and neuroprotective effects [35]. In DIO mice, lycopene was able to suppress body weight gain, block lipid accumulation in adipose tissue, and improve insulin resistance in white adipose tissue (WAT) [36]. Lycopene has also been shown to attenuate inflammation and insulin resistance in the epididymal WAT and liver of HFD-fed obese mice by facilitating adipose tissue macrophage polarization [37]. Another carotenoid, β-cryptoxanthin, reduced body fat gain and plasma glucose levels in HFD-fed obese mice while increasing energy expenditure [38].
Celastrol, a plant-derived quinone methide triterpenoid, reportedly possesses preventative and therapeutic properties in attenuating metabolic dysregulations like obesity through anti-oxidative and anti-inflammatory activities [39]. Additionally, celastrol administration enhanced systemic insulin sensitivity in HFD-fed obese rats, attenuated inflammatory responses and macrophage polarization in adipose tissues, improved skeletal muscle mitochondrial functions, and stimulated muscle mitochondrial biogenesis. In HFD-obese mice, celastrol has also been able to reduce intensinal lipid transporters and absorption; findings from this study also suggest that gut microbiota composition is critical in mediating the anti-obesity effects of celastrol [40].
Outlook and Resources
The milestone efficacies achieved by semaglutide and other newly-developed anti-obesity medications will hopefully encourage the further development of effective therapies against obesity. With these advancements, it is more likely than ever that pharmacological therapies will achieve comparable efficacy with weight loss surgeries in the future. Although preclinical research has led to groundbreaking discoveries in this field, clinical testing, especially in diverse populations, will be required to evaluate drug efficacy and safety in humans.
For further insights, check out our 2020 Obesity Webinar Series as well as our most recent podcasts and webinars on late-breaking obesity research.
About the Author
About the Author

Nina Culum graduated from the University of Western Ontario with a Master of Science in physical and analytical chemistry. During her graduate studies, she fabricated plasmonic nanohole arrays to capture extracellular vesicles and detect cancer by surface-enhanced Raman spectroscopy. Prior to attending UWO, Nina completed her Bachelor of Science in chemistry at the University of Waterloo.
References
- World Health Organization [Internet]. Obesity and overweight; 2021 Jun 09 [cited 2022 Feb 24]. Available from: https://www.who.int/news-room/fact-sheets/detail/obesity-and-overweight.
- Kleinert M, Clemmensen C, Hofmann SM, Moore MC, Renner S, et al. Animal models of obesity and diabetes mellitus. Nat Rev Endocrinol. 2018;14(3):140-62. DOI: 10.1038/nrendo.2017.161.
- Centers for Disease Control and Prevention [Internet]. Adult obesity causes & consequences; 2021 Mar 22 [cited 2022 Mar 02]. Available from: https://www.cdc.gov/obesity/adult/causes.html.
- Müller TD, Blüher M, Tschöp MH, DiMarchi RD. Anti-obesity drug discovery: advances and challenges. Nat Rev Drug Discov. 2021. DOI: 10.1038/s41573-021-00337-8. Online ahead of print.
- Ruban A, Stoenchev K, Ashrafian H, Teare J. Current treatments for obesity. Clin Med (Lond). 2019;19(3):205-12. DOI: 10.7861/clinmedicine.19-3-205.
- Food and Drug Administration [Internet]. FDA approves new drug treatment for chronic weight management, first since 2014; 2021 Jun 04 [cited 2022 Mar 2]. Available from: https://www.fda.gov/news-events/press-announcements/fda-approves-new-drug-treatment-chronic-weight-management-first-2014.
- Wilding JPH, Batterham RL, Calanna S, Davies M, Van Gaal LF, et al. Once-weekly semaglutide in adults with overweight or obesity. N Engl J Med. 2021;384(11):989. DOI: 10.1056/NEJMoa2032183.
- Preguiça I, Alves A, Nunes S, Fernandes R, Gomes P, et al. Diet-induced rodent models of obesity-related metabolic disorders-a guide to a translational perspective. Obes Rev. 2020;21(12):e13081. DOI: 10.1111/obr.13081.
- Speakman JR. Measuring energy metabolism in the mouse – theoretical, practical, and analytical considerations. Front Physiol. 2013;4:34. DOI: 10.3389/fphys.2013.00034.
- Lighton JRB. Limitations and requirements for measuring metabolic rates: a mini review. Eur J Clin Nutr. 2017;71(3):301-5. DOI: 10.1038/ejcn.2016.265.
- Columbus Instruments. CLAMS Comprehensive Lab Animal Monitoring System: Oxymax®-CLAMS. [cited 2022 Mar 10]. Available from: https://www.colinst.com/products/clams-comprehensive-lab-animal-monitoring-system
- Schafer MJ, Mazula DL, Brown AK, White TA, Atkinson E, et al. Late-life time-restricted feeding and exercise differentially alter healthspan in obesity. Aging Cell. 2019;18(4):e12966. DOI: 10.1111/acel.12966.
- Zhang D, Lee JH, Shin HE, Kwak SE, Bae JH, et al. The effects of exercise and restriction of sugar-sweetened beverages on muscle function and autophagy regulation in high-fat high-sucrose-fed obesity mice. Diabetes Metab J. 2021;45(5):773-86. DOI: 10.4093/dmj.2020.0157.
- Chae SA, Son JS, Zhu M-J, De Avila JM, Du AM. Treadmill running of mouse as a model for studying influence of maternal exercise on offspring. Bio Protoc. 2020;10(23):e3838. DOI: 10.21769/BioProtoc.3838.
- Manzanares G, Brito-da-Silva G, Gandra PG. Voluntary wheel running: patterns and physiological effects in mice. Braz J Med Biol Res. 2019;52(1):e7830. DOI: 10.1590/1414-431X20187830.
- Nigro P, Middelbeek RJW, Alves CRR, Rovira-Llopis S, Ramachandran K, et al. Exercise training promotes sex-specific adaptations in mouse inguinal white adipose tissue. Diabetes. 2021;70(6):1250-64. DOI: 10.2337/db20-0790.
- Chen VP, Gao Y, Geng L, Brimijoin S. Butyrylcholinesterase gene transfer in obese mice prevents postdieting body weight rebound by suppressing ghrelin signaling. Proc Natl Acad Sci USA. 2017;114(41):10960-5. DOI: 10.1073/pnas.1706517114.
- Zou J, Lai B, Zheng M, Chen Q, Jiang S, et al. CD4+ T cells memorize obesity and promote weight regain. Cell Mol Immunol. 2018;15(6):630-9. DOI: 10.1038/cmi.2017.36.
- Mroz PA, Finan B, Gelfanov V, Yang B, Tschöp MH, et al. Optimized GIP analogs promote body weight lowering in mice through GIPR agonism not antagonism. Mol Metab. 2019;20:51-62. DOI: 10.1016/j.molmet.2018.12.001.
- Finan B, Ma T, Ottaway N, Müller TD, Habegger KM, et al. Unimolecular dual incretins maximize metabolic benefits in rodents, monkeys, and humans. Sci Transl Med. 2013;5(209):209ra151. DOI: 10.1126/scitranslmed.3007218.
- Finan B, Yang B, Ottaway N, Smiley DL, Ma T, et al. A rationally designed monomeric peptide triagonist corrects obesity and diabetes in rodents. Nat Med. 2015;21(1):27-36. DOI: 10.1038/nm.3761.
- Perry RJ, Zhang D, Zhang X-M, Boyer JL, Shulman GI. Controlled-release mitochondrial protonophore reverses diabetes and steatohepatitis in rats. Science. 2015;347(6227):1253-6. DOI: 10.1126/science.aaa0672.
- Alexopoulos SJ, Chen S-Y, Brandon AE, Salamoun JM, Byrne FL, et al. Mitochondrial uncoupler BAM15 reverses diet-induced obesity and insulin resistance in mice. Nat Commun. 2020;11:2397. DOI: 10.1038/s41467-020-16298-2.
- Axelrod CL, King WT, Davuluri G, Noland RC, Hall J, et al. BAM15‐mediated mitochondrial uncoupling protects against obesity and improves glycemic control. EMBO Mol Med. 2020;12(7):e12088. DOI: 10.15252/emmm.202012088.
- Mullican SE, Lin-Schmidt X, Chin C-N, Chavez JA, Furman JL, et al. GFRAL is the receptor for GDF15 and the ligand promotes weight loss in mice and nonhuman primates. Nat Med. 2017;23(10)1150-7. DOI: 10.1038/nm.4392.
- Poulsen C, Østergaard Pedersen M, Wahlund P-O, Sjölander A, Kaalby J, et al. Rational Development of Stable PYY3–36 Peptide Y2 Receptor Agonists. Pharm Res. 2021;38(8):1369-85. DOI: 10.1007/s11095-021-03077-x.
- Murugan DD, Balan D, Wong P-F. Adipogenesis and therapeutic potentials of antiobesogenic phytochemicals: insights from preclinical studies. Phytother Res. 2021;35(11):5936-60. DOI: 10.1002/ptr.7205.
- De Santis Stefania ML, Clodoveo M, Cariello G, D’Amato C, Franchini MF, et al. Polyphenols and obesity prevention: critical insights on molecular regulation, bioavailability and dose in preclinical and clinical settings. Crit Rev Food Sci Nutr. 2021;61(11):1804-26. DOI: 10.1080/10408398.2020.1765736.
- Choi LS, Jo IG, Kang KS, Im JH, Kim J, et al. Discovery and preclinical efficacy of HSG4112, a synthetic structural analog of glabridin, for the treatment of obesity. Int J Obes (Lond), 2021;45:130-42. DOI: 10.1038/s41366-020-00686-1.
- Luna-Vital D, Luzardo-Ocampo I, Cuellar-Nuñez L, Loarca-Piña G, Gonzalez de Mejia E. Maize extract rich in ferulic acid and anthocyanins prevents high-fat-induced obesity in mice by modulating SIRT1, AMPK and IL-6 associated metabolic and inflammatory pathways. J Nutr Biochem. 2020;79:108343. DOI: 10.1016/j.jnutbio.2020.108343.
- Shabani M, Sadeghi A, Hosseini H, Teimouri M, Khorzoughi RB, et al. Resveratrol alleviates obesity-induced skeletal muscle inflammation via decreasing M1 macrophage polarization and increasing the regulatory T cell population. Sci Rep. 2020;10:3791. DOI: 10.1038/s41598-020-60185-1.
- Pericardo DJ, Rodriguez Lanzi C, Gambarte Tudela J, Miatello RM, Oteiza PI. Quercetin attenuates adipose hypertrophy, in part through activation of adipogenesis in rats fed a high-fat diet. J Nutr Biochem. 2020;79:108352. DOI: 10.1016/j.jnutbio.2020.108352.
- Hwang D, Seo J-B, Park H-Y, Kim J, Lim K. Capsiate intake with exercise training additively reduces fat deposition in mice on a high-fat diet, but not without exercise. Int J Mol Sci. 2021;22(2):769. DOI: 10.3390/ijms22020769.
- Xu M, Yang L, Zhu Y, Liao M, Chu L, et al. Collaborative effects of chlorogenic acid and caffeine on lipid metabolism via the AMPKα-LXRα/SREBP-1c pathway in high-fat diet-induced obese mice. Food Funct. 2019;10(11):7489-97. DOI: 10.1039/C9FO00502A.
- Wang J, Li L, Wang Z, Cui Y, Tan X, et al. Supplementation of lycopene attenuates lipopolysaccharide-induced amyloidogenesis and cognitive impairments via mediating neuroinflammation and oxidative stress. J Nutri Biochem. 2018;56:16-25. DOI: 10.1016/j.jnutbio.2018.01.009.
- Wang J, Suo Y, Zhang J, Zou Q, Tan X, et al. Lycopene supplementation attenuates western diet-induced body weight gain through increasing the expressions of thermogenic/mitochondrial functional genes and improving insulin resistance in the adipose tissue of obese mice. J Nutr Biochem. 2019;69:63-72. DOI: 10.1016/j.jnutbio.2019.03.008.
- Chen G, Ni Y, Nagata N, Zhuge F, Xu L, et al. Lycopene Alleviates Obesity-Induced Inflammation and Insulin Resistance by Regulating M1/M2 Status of Macrophages. Mol Nutr Food Res. 2019;63(21):e1900602. DOI: 10.1002/mnfr.201900602.
- Hara H, Takahashi H, Mohri S, Murakami H, Kawarasaki S, et al. β-cryptoxanthin induces UCP-1 expression via a RAR pathway in adipose tissue. J Agric Food Chem. 2019;67(35):10595-603. DOI: 10.1021/acs.jafc.9b01930.
- Abu Bakar MH, Shariff KA, Tan JS, Lee LK. Celastrol attenuates inflammatory responses in adipose tissues and improves skeletal muscle mitochondrial functions in high fat diet-induced obese rats via upregulation of AMPK/SIRT1 signaling pathways. Eur J Pharmacol. 2020;883:173371. DOI: 10.1016/j.ejphar.2020.173371.
- Hua H, Zhang Y, Zhao F, Chen K, Wu T, et al. Celastrol inhibits intestinal lipid absorption by reprofiling the gut microbiota to attenuate high-fat diet-induced obesity. iScience. 2021;24(2):102077. DOI: 10.1016/j.isci.2021.102077.