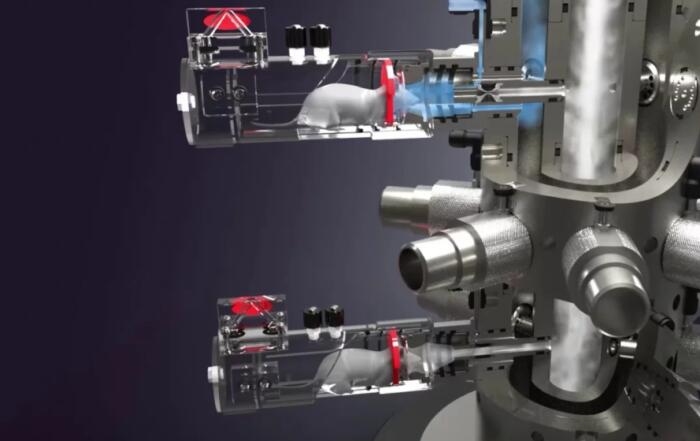
In this webinar, Dr. Valeria Fumagalli and Dr. Nancy Mounogou Kouassi present two novel models of COVID-19: golden hamsters and aerosolized SARS-CoV-2 delivery in mice.
Highlights
- Aerosolized SARS-CoV-2 delivery in transgenic mice
- At risk populations for severe influenza infections
- Syrian golden hamsters as a model for COVID-19
Webinar Summary
Dr. Fumagalli begins this webinar with a brief introduction to current models of SARS-CoV-2 and their limitations. K18-hACE2 transgenic mice have been widely used to study the effects and pathophysiology of COVID-19, with the infection typically being induced via intranasal injection. However, this methodology does not accurately replicate what happens in clinical settings, as these mice typically die within a week of inoculation from an infection in the central nervous system. These limitations are rather difficult to ignore, as they greatly limit their usefulness in studying disease pathogenesis and potential treatments.
To determine whether the K18-hACE2 transgenic mouse lineage could still be useful for modeling SARS-CoV-2, Dr. Fumagalli wanted to more accurately replicate the way that infections spread in the real world by nebulizing the virus and delivering it to anesthetized mice through the nasal cavity. This delivery method greatly improved mouse mortality, and no traces of viral RNA were found in their brains. Additionally, the viral RNA found in their lungs was comparable to that observed in conventional models, indicating that this novel model was effective in inducing the disease. Dr. Fumagalli hopes to use this method of viral delivery with a new mouse model so as to better replicate the long-term sequelae of COVID-19.
In the second half of this webinar, Dr. Kouassi discusses SARS-CoV-2 pathogenesis in a Syrian golden hamster model and highlights the sex-dependent differences of cell immunity. Prior to the COVID-19 pandemic, Dr. Kouassi primarily focused on identifying at-risk populations for influenza, and determining how sex hormones can impact the lung’s ability to recover post-infection. Since the pandemic, she has shifted her focus to SARS-CoV-2 with the Syrian golden hamster as a model.
“Females of reproductive age are more likely to be hospitalized [and] develop severe influenza infection than men.”
Dr. Kouassi sought to characterize the sex differences in SARS-CoV-2 pathogenesis using this novel model to determine the differential effect of cell immunity. Lung function was more impaired in male hamsters than female hamsters, which was likely due to greater CYP19A1 expression in the male lung. CYP19A1, which translates to the enzyme aromatase, an estrogen synthase, aromatizes androgens into estrogens and is increasingly transcribed upon SARS-CoV-2 infection. Letrozole, an aromatase inhibitor, was shown to improve lung function in male hamsters after 21 days, but had no effect on lung function in female hamsters, further supporting this mechanism. Additionally, elevated CYP19A1 expression has been found in the lungs of men with COVID-19, indicating that this sex-dependent mechanism is evolutionarily shared.
In summary, while many of the short-term effects of COVID-19 are being increasingly understood, the long-term sequelae have still yet to be characterized. Preclinical models that better represent disease pathophysiology will help further this process, and will hopefully lead to a cure for the long-term effects of SARS-CoV-2 infection.
Click to watch the webinar recording. To view the presentation full screen simply click the square icon located in the bottom-right corner of the video viewer.
Resources
Q&A
- Why did you choose to perform these experiments on Syrian golden hamsters?
- Can you share your experience with animal handling in the system restrainer?
- What are some advantages of using unrestrained animals?
- Did you get a chance to look at the survival rate of the K18-hACE2 mice?
- Do transgenic ACE2 mice challenged with SARS-CoV-2 die from specifically neurological infection?
- Does letrozole work in castrated hamsters?
- How were you measuring the amount of virus that reached the lungs when administered via the inhalation tower?
- Does aerosolization reduce the infectivity of SARS-CoV-2?
- Do you think these models would be useful for studying long COVID?
To retrieve a PDF copy of the presentation, click on the link below the slide player. From this page, click on the “Download” link to retrieve the file.
Presenters
Postdoctoral Fellow
Dynamics of Immune Responses
San Raffaele University
Postdoctoral Fellow
Viral Zoonoses - One Health
Leibniz Institute of Virology