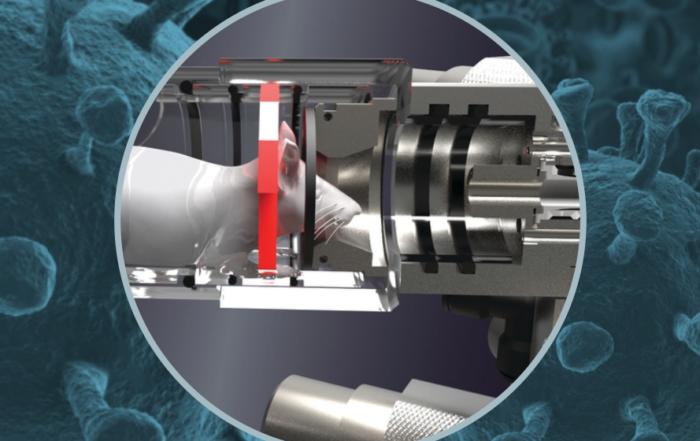
UV Shemesh presents Buxco's nose-only rodent inhalation system, highlighting how it can improve research efficiency and offer valuable insights into inhalation respiratory studies.
Inhalation delivery remains the preferred pre-clinical choice as it provides fast route to the blood stream with minimal side effects. However, the research itself contains several challenges that turns many researchers away from this delivery method. Over the past couple of years, several features have been added to Buxco’s nose-only inhalation system, improving on its flexibility and study optimization tools. In this webinar, UV Shemesh introduces those features, discusses their practical benefits and applications, and demonstrates how they can simplify inhalation research while gaining important insights and efficiency.
Key Topics Include:
- Nebulizer calibration and regulation to ensure more reproducible aerosol deliveries
- Integration of plethysmography with animal restraint to optimize flow rate and contribute to long-term compound savings
- Tips to assess and optimize the tolerability of vehicle and test compounds using plethysmography
- How automated aerosol characterization can simplify the identification of optimal system conditions
- How gravimetric collection techniques can replace live digital aerosol concentration measurements to improve real-time dose reporting
- The use of delivered dose reports and respiratory endpoints to serve as predictive indicators for lung deposition through imaging technology
- Overview of SmartStudy’s capabilities, including:
- Automated aerosol delivery based on user-defined targets for dose or time
- Management of single or multiple-dosage groups
- Conducting T0 sacrifices without compromising chamber environment by staggering duration protocol
Click to watch the webinar recording. To view the presentation full screen simply click the square icon located in the bottom-right corner of the video-viewer.
Resources
Presenters
Senior Product Manager
Inhalation and Respiratory
Data Sciences International
UV Shemesh has 27 years of experience within the pre-clinical respiratory and inhalation space, starting his career off at Buxco Research Systems before joining DSI as chief engineer and now in a product management role. He has supported many researchers in their studies using the Buxco systems, and we are thrilled to have him share some of those experiences with us.
Production Partner
Data Sciences International
Data Sciences International (DSI) is the leader in preclinical physiological monitoring offering telemetry, instrumentation, software and services to help advance science. Industries served include: Pharmaceuticals, Academia, Contract Research Organizations, Biological and Chemical Defense, Medical Devices, Government, and Biotechnology.
Harvard Bioscience, Inc.
Harvard Bioscience is a global leader in the manufacturing and distribution of solutions to advance life science research. For over 110 years, we have served the changing needs of life scientists in over 100 countries. Our expanding portfolio of brands include instruments for organ and animal research, cell analysis, molecular biology, fluidics, and laboratory consumables.
Additional Content From Data Sciences International
Novel Animal Models and Methods for Studying COVID-19: Golden Hamsters and Aerosolized SARS-CoV-2 Delivery
Experts present two novel animal models of COVID-19 and highlight recent research conducted using these models.
Assessing Toxicity and Health Risks of E-cigarettes: How to Take Aim at a Moving Target
Dr. Emma Karey presents research that challenges the convention that extant cigarette endpoints sufficiently capture the health risks of vaping, and provides a translational framework for how to integrate scientific principles and novel conditions.
Continuous Glucose Monitoring and Glycemic Variability in Pregnancy
Join Caroline Wuyts, MSc for a deep dive into her research on pregnancy and monitoring glucose availability throughout the reproductive timeline of a mouse model.
Additional Content From Harvard Bioscience, Inc.
Mapping Heterogeneous Interfaces Using Single-Entity Electrochemical Microspectroscopy
Dr. Venky Prabhakaran describes the development of scanning electrochemical microspectroscopy and its application to characterize heterogeneous electrified interfaces.
Organoid Meets Microelectrode Array (MEA) – Accelerating Drug Discovery and Development
In this webinar, Dr. Sven Schönecker and Sara Mirsadeghi, MSc, will discuss 3D Mesh Microelectrode Arrays (MEA) including new developments and applications.
Molecule Transport across Cell Membranes: Electrochemical Quantification at the Microscale
In this webinar, Dr. Sabine Kuss discusses the importance of transmembrane molecule exchange and how to detect and quantify membrane transport of molecules in cells.
Related Content
Preclinical Animal Models of IBD, GVHD, and Pulmonary Disease
Join BioModels’ Scientists, Andrew Borkowski, PhD and Nicole Smith, PhD, as they discuss a selection of key preclinical models of inflammatory disease, including models of Inflammatory Bowel Disease (IBD)/colitis, Graft vs Host Disease (GvHD), and pulmonary disease, focusing on the utility of clinically relevant endpoints that create highly translational models of human disease.
Next-Generation Safety Assessment Tools for Advancing In Vivo to In Vitro Translation
In this webinar, Dr. Louis Scott discusses the morph_ONE™ assay, a novel tool for in vitro safety assessment in drug development.
Visualizing the Intracellular Immune Response to SARS-CoV-2 with Fast, High-Content Imaging
Experts discuss how automated microscopy and image analysis can quantify critical markers of infection and describe the insight provided by microscopy in the context of SARS-CoV-2 infection and inflammation.