Can the Immune System Protect after Repeated Myocardial Injury?
Blog post by Jasmin Skinner
Regardless of the cause and specific etiology, cardiomyopathies impair the heart’s ability to pump blood (1). Possible consequences include arrhythmia, heart failure, and death, and in some instances a recurrence of cardiac injury can occur. This may result in a chronically enlarged heart, known as dilated cardiomyopathy (2). While the immune response to a single cardiomyopathic injury has been extensively studied, the immune response after repeated injury has not been as thoroughly characterized (3). If the immune response to recurring cardiomyopathy can adapt and reduce the effects of repeated cardiac injury, it may represent a potential target for preventative treatment in high risk individuals. A recent publication from Tiwary et al., investigated this pathway in a murine model, and is discussed in this blog (3).
This webinar features Artur Fedorowski, MD, PhD, as he describes the relationship between cardiovascular dysautonomia after COVID-19, and the diagnostics behind detecting Long COVID or Post-acute Sequalae of COVID-19. WATCH HERE.
Study parameters and repeated cardiac injury protocol
To determine whether the mouse immune system is capable of adapting and mounting a cytoprotective response to repeated cardiac injury, 10 week old male and female mice were injected with isoproterenol (ISO), a drug used to treat bradycardia conditions that causes increased heart rate and contractility, peripheral vasodilation, and relaxation of the bronchial, gastrointestinal, and uterine smooth muscle (4); control mice received vehicle injections. All mice were allowed to recover for 7 days after injection, before receiving a second dose of either ISO or the vehicle solution. Additionally, to determine whether this cytoprotective response was maintained, they reinjected the ISO-pretreated mice with a second ISO injection 21 and 42 days after the initial injection (3).
To assess the severity of cardiac injury, Evans blue dye uptake and serum troponin I levels were monitored. Troponin, as well as other biomolecules, is released when the heart muscle has been damaged, making it an excellent indicator to determine whether ISO-induced cardiac injury is lessened after pre-exposure. Additionally, 2D echocardiographic studies were performed to determine left ventricular (LV) regional wall motion, and LV contractility by Langendorff perfusion (3).
Prior cardiac tissue injury protected the heart from subsequent damage
Previous isoproterenol-induced tissue injury was effective at protecting the heart from subsequent myopathic effects after repeated exposure, as determined by decreased inflammation and ISO-induced cardiac myocyte cell death, and preservation of left ventricular structure and function. The ISO treated mice also had improved chances of survival, providing clear evidence that prior exposure to ISO can improve health outcomes in a murine model of repeated cardiac injury. These cytoprotective responses persisted for 2 weeks post-injection, but after 5 weeks the cytoprotective response was partly diminished, as mice re-exposed at this time had an increase in troponin release, Ly6G+ neutrophils, and CD64+ macrophages. However, despite this reduced cytoprotection, the benefits of ISO-induced preconditioning still preserved overall myocardial homeostasis after an additional injection at 5 weeks, as no significant change in left ventricular function or dilation was observed (3).
To determine whether these protective effects are due to the immune response from the initial injury, macrophages were depleted in ISO-preconditioned hearts using liposomes containing clodronate, a drug commonly used to prevent bone metastases in breast cancer patients (5). These clodronate-liposomes deplete resident macrophages, resulting in reduced cytoprotection and increased ISO-induced cardiac myocyte cell death (3). This observation demonstrates that the innate immune response was capable of reducing the subsequent tissue damage from an additional exposure to ISO, and is consistent with the possibility that immune-mediated tissue repair mechanisms may provide tolerance to additional tissue damage, regardless of the cause (3).
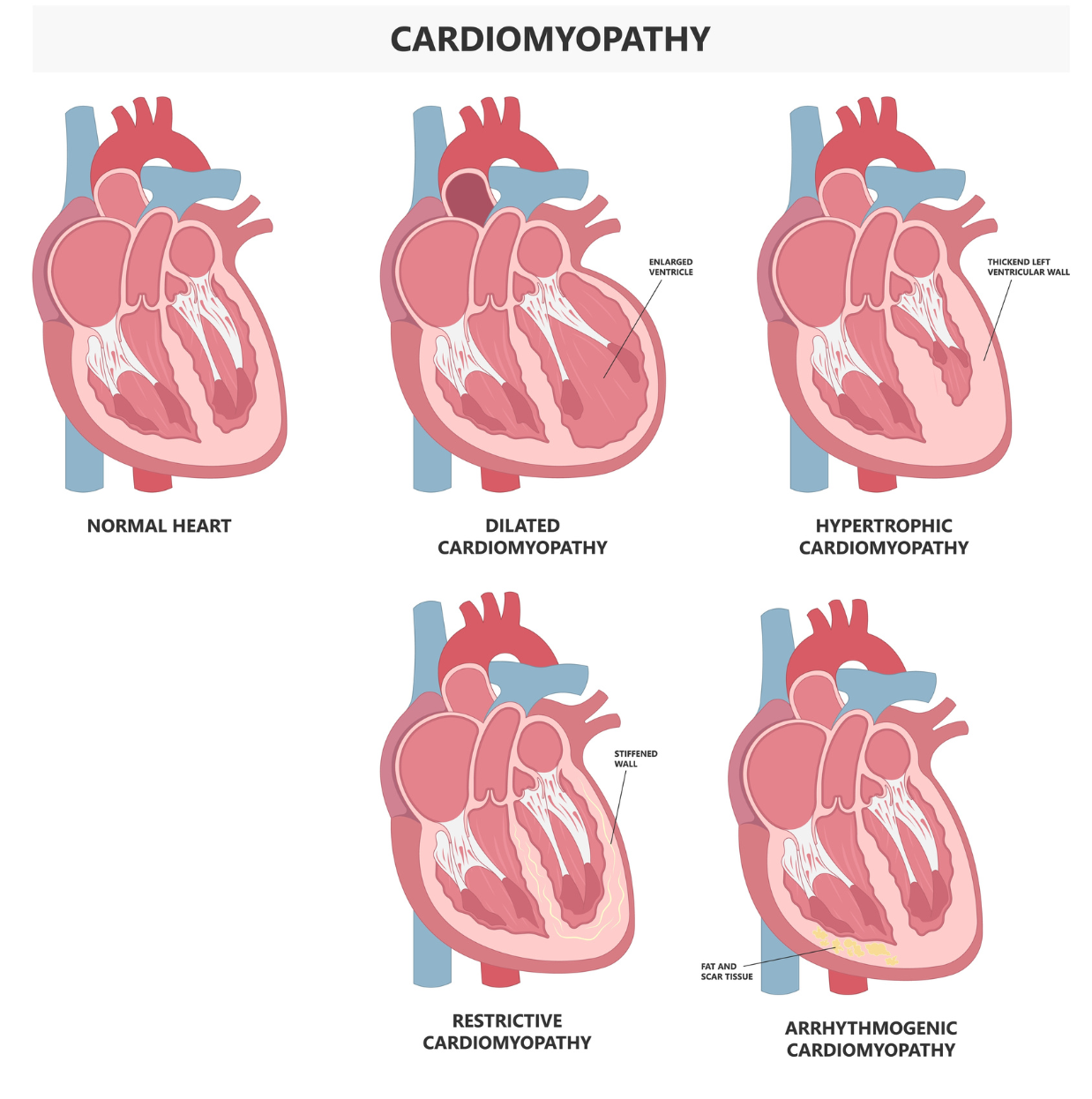
Figure 1: The five main types of cardiomyopathies. The ISO-induced cardiomyopathy exhibits similar characteristics to dilated and hypertrophic cardiomyopathies. Image used under license from Shutterstock.com.
Study limitations and future directions
While this study highlights the potential of preventative treatment for cardiomyopathies, additional research is required to validate these findings in human patients. However, the present study did support the suggestion that cytoprotection of the heart muscles is analogous to disease tolerance, indicating that the immune system is capable of attenuating future ISO-induced tissue damage by limiting myocyte cell death and preserving myocardial homeostasis. However, this study did not address whether these cytoprotective effects occur after cytokine/chemokine release by macrophages, or via a direct interaction between cardiomyocytes and macrophages. Regardless of these immuno-mechanics, the authors suggest that future efforts may benefit from focusing on delineating mechanisms that restore tissue homeostasis, rather than continue to identify novel preconditioning stimuli (3).
About the Author
About the Author

Jasmin Skinner is an undergraduate student at the University of Western Ontario completing a Specialization in Biology and a Minor in Chemistry, with focused interest in applying these concepts to environmental conservation. As a lover of the outdoors and the arts, much of her time is spent in nature and within the local London art community, creating and connecting with all walks of life. After graduating, she hopes to continue her passion of finding unconventional solutions to environmental issues by working with nature, not against it.
References
- John Hopkins Medicine [Internet]. Maryland:Johns Hopkins HealthCare LLC; [date unknown]. Cardiomyopathy; [date unknown] [cited 2023 April]; [Health > Conditions and Diseases]. Available from: https://www.hopkinsmedicine.org/health/conditions-and-diseases/cardiomyopathy
- About myocarditis [Internet]. Kingwood (TX): [publisher unknown]; [date unknown; cited 2023 April]. Available from: https://www.myocarditisfoundation.org/about-myocarditis/#:~:text=Yes%2C%20myocarditis%20can%20recur%2C%20and,to%20prevent%20recurrence%20of%20myocarditis.
- Tiwary SK, Hayashi T, Kovacs A, Mann DL. Recurrent myocardial injury leads to disease tolerance in a murine model of stress-induced cardiomyopathy. JACC Basic Transl Sci [Internet]. Forthcoming 2023 [cited 2023 April]. Available from: https://www.jacc.org/doi/10.1016/j.jacbts.2022.12.007. doi: 10.1016/j.jacbts.2022.12.007
- Szymanski MW, Singh DP. Isoproterenol. [eBook]. Treasure Island (FL): StatPearls Publishing; 2023 Jan. Available from: StatPearls
- Dando TM, Wiseman LR. Clodronate : a review of its use in the prevention of bone metastases and the management of skeletal complications associated with bone metastases in patients with breast cancer. Drugs Aging [Internet]. 2004 [cited 2023 April];21(14):949-69. Available from: https://pubmed.ncbi.nlm.nih.gov/15554753/. doi: 10.2165/00002512-200421140-00005