Q&A Report: Turning Innovative Features Into Practical Benefits Within the Buxco Nose-Only Rodent Inhalation System
The answers to these questions have been provided by:
UV Shemesh
Senior Product Manager
Inhalation and Respiratory
Data Sciences International
For gravimetric collection, what if particles are so small they do not get caught by the filter?
There are various filter membranes that have different pore sizes that can be used with our gravimetric filter, depending on the specific aerosol properties. Filter material should also be considered.
Will the estimated time be adjusted to the fixed aerosol concentration you set, and why is the fixed aerosol concentration your preferred method? What is the use of the photometer in this case?
The estimated time will not be adjusted to the fixed aerosol concentration; that is calculated based on mathematical calculation of solution concentration, flows, and nebulizer output. I prefer that method since it has the option to calculate only API, removing the vehicle, so AIA shows more relevant data without the need for post-analysis. In this case, the photometer is used as a reference. It’s certainly not required if using the fixed concentration feature, but it’s nice to have a digital readout as it provides a data point on the aerosolization itself.
Thanks a lot for this fantastic talk! Is it necessary to disassemble the inhalation tower and clean the chamber every time we want to move to another control group with different formulations? Is there a simple way to clean the chamber?
Thank you! In general, yes, it’s recommended to clean the tower and front-end of the chambers (restrainers) when changing formulation. The tower disassembles very quickly, without tools, and can be easily cleaned, sprayed, or autoclaved. The front-end piece of the restrainer is exposed to aerosol and should be cleaned, typically with less harsh materials. Depending on the aerosol itself, spraying with the proper agents has been quick and effective.
Interesting way of controlling the system to achieve precise dosing to each animal. How do we clean the system (In particular the valve that controls the delivery of the aerosol to the animal) to ensure that there is no cross-contamination between studies?
Thanks! See above answer for cleaning, but particularly to the jet valve (silicone bladder), it’s autoclavable as well, as a single assembled piece. If the material is of sticky nature, it can be cleaned with a thin brush.
Can the software control multiple exposure chambers concurrently or do you need a laptop for each exposure chamber?
Multiple instances of the software can be run at the same time, so you do NOT need a laptop for each exposure chamber.
I have a question about smart technology. How does that actually work and specifically where does the fresh air come from?
We utilize clean compressed air connected to the controller, which is then splits and controls the SmartStudy ports. The high pressure closes the SmartStudy port via a bladder, and also supplies the fresh air via a critical orifice technology.
Thank you for the webinar, very informative. You seemed to focus on pharma research a lot. Does the system also support other applications such as smoke, gases, and others?
Yes, the system is suitable for all types of application and aerosols, although certain features, such as SmartStudy, are only applicable to specific ones.
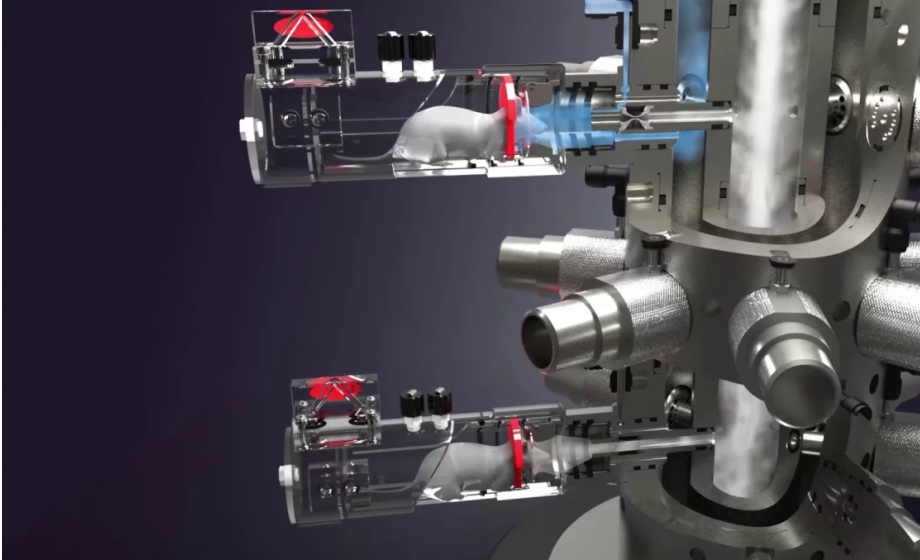
How do respiratory endpoints get measured during exposure? What kind of chamber is used, and doesn’t that complicate the usage of the system?
Our specification is +/-8%, which is tested in our IQ/OQ processes. The chamber used is a head-out plethysmograph design, which is direct flow. It complicates the system a little, but we have made it as easy as possible with a single click calibration process. The data from the respiratory endpoints make the extra step well worth it.
How accurate is the ventilation measurement of the system, as it seems to be an integral part of the system?
See answer above.
We’ve been having issues with pressure management in our inhalation system. Can you speak on how this system achieved a slight negative pressure?
Our system runs a PID regulation on the negative system flow to regulate to a user-defined pressure. The InFlow (positive) which supplies the fresh air to the animals is static regulation.
Could you elaborate more on how we can assess the tolerability of new test compounds using plethysmography?
Respiratory endpoints is a good sign, and a clever way to leverage SmartStudy is to compare control vs exposed subject at the same time by closing one or more ports and assessing the respiratory reaction to the aerosol.
Do the delivered dose reports and respiratory endpoints provided by Buxco correlate well with actual lung deposition confirmed through imaging techniques?
It’s too early to make a general statement, as the data is just starting to come in. There are certainly good and exciting signs, and I will keep this audience in the loop as publications come out.
What’s the suitability for long-term studies, and especially to ensure results stay consistent over extended periods?
The regulation feature of the system, specifically the nebulizer output, and pressure regulation, are highly suitable for consistent usage of extended periods. The automated mitigation of the equipment degradation really helps as well the removal of user error.
From a broader perspective, how do you see the future of inhalation studies evolving?
I believe inhalation is a good place with new applications and needs popping up (wildfires exposure)… I do believe that there’s a need to reduce animal count while achieving similar results and a need to simplify the inhalation exposure experience, so more and more labs stay and enter this field vs other routes of delivery.