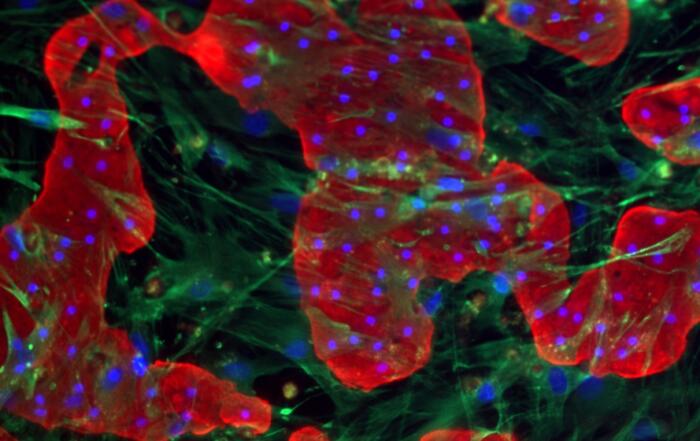
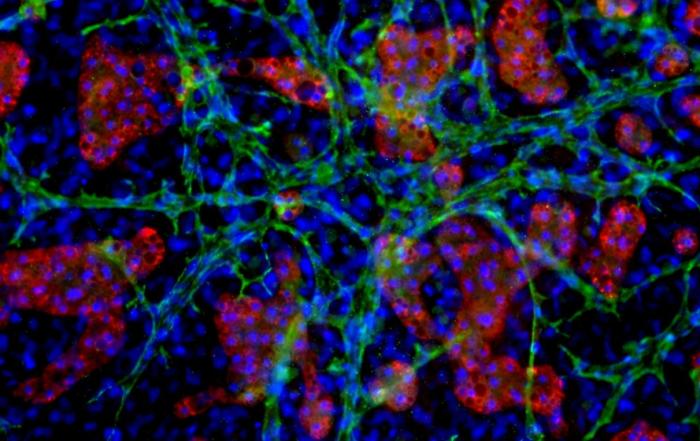
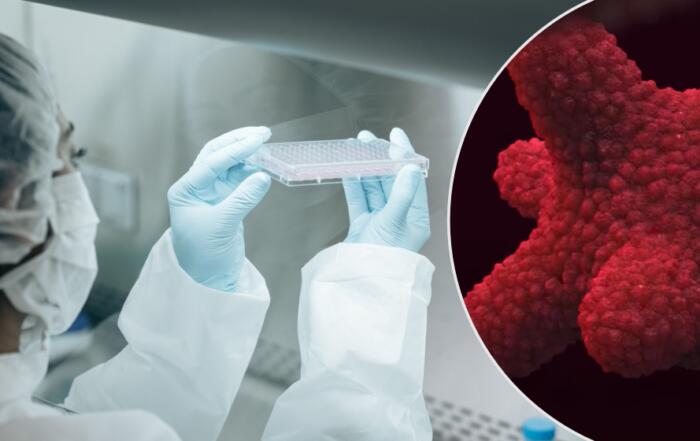
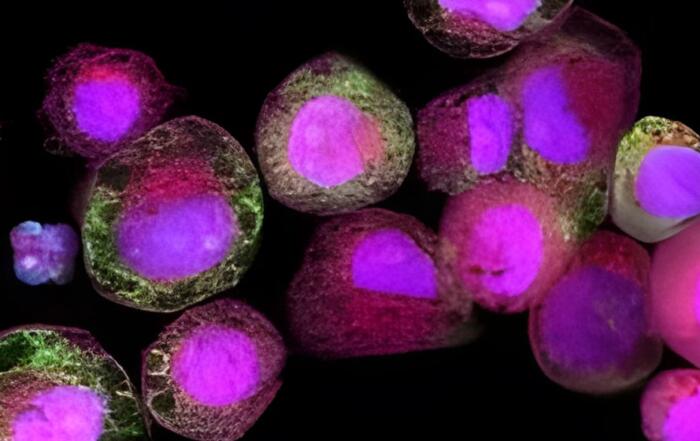
Dr. Chad Deisenroth, PhD, from the US EPA, introduces an advanced human in vitro 3D thyroid microtissue model designed to evaluate thyroid hormone disruption, addressing current challenges with existing models. Developed in collaboration with LifeNet Health, this model offers hormonogenic competence and the capacity to connect mechanistic insights with functional outcomes like thyroid hormone synthesis.
In this webinar, Dr. Deisenroth will discuss the US EPA Endocrine Disruptor Screening Program’s (EDSP) approach to screen chemicals for potential thyroid-disrupting effects and the urgent need to advance new approach methods that protect human health while reducing animal testing. He will showcase how hormone production in the model mimics human endocrine biology and is inhibited following exposure to known reference chemicals that affect key targets within the thyroid gland.
Dr. Deisenroth will also explain the methodology behind establishing minimum donor qualification criteria and benchmark parameters for test method standardization, transferability, and validation. This approach balances performance standardization with the ability to predict a range of human responses.
Lindsey Whaley from LifeNet Health will unveil the first primary human thyrocyte product that enables access to the 3D thyroid microtissue model. Join us for a journey into cutting-edge scientific research that promises to reshape how we assess thyroid disruption.
Key Topics Include:
- Development of the 3D thyroid microtissue model.
- Evaluation of model performance, including the production of thyroid hormone.
- Standardization of the model for thyroid disruption testing.
- Categories: Cell & Molecular Biology, Drug Discovery & Development, Pharmacology, Toxicology
- Tags: cell metabolism, drug development, drug discovery, drug metabolism, drug testing, drug-drug interaction, human biological sample (HBS), human cells, Mammalian cell culture, pharmacokinetics, pharmacology, safety pharmacology, toxicology
Presenters
Chad Deisenroth, PhD
Cell Biologist
Center for Computational Toxicology & Exposure
U.S. Environmental Protection Agency
Lindsey Whaley, MS
Product Manager
LifeSciences
LifeNet Health