Tick Tock: The Growing Risk of Lyme Disease and Efforts to Develop a Vaccine
Blog post by Jasmin Skinner
Ticks are the leading cause of zoonotic pathogens and are reported to carry over 16 different infectious agents and bacteria within North America (1,2). These bacteria include Borrelia burgdorferi, better known as the cause of Lyme disease (1,3). The CDC recently estimated 476,000 individuals are diagnosed with and treated for Lyme disease in the United States annually, and it is clear that this bacteria is of increasing public health concern (1).
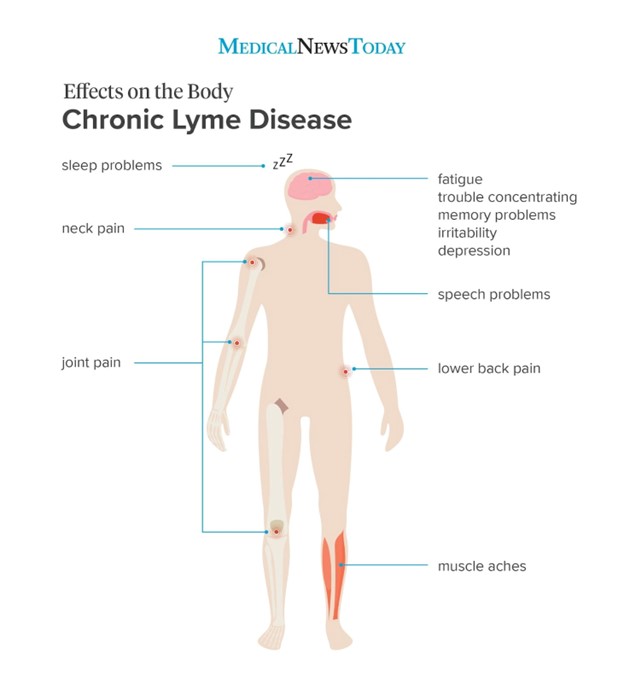
The early signs and symptoms can vary between individuals but typically include a characteristic bulls-eye rash, fever, chills, headache, joint pain, and swollen lymph nodes (4). However, these symptoms are not consistent in all individuals and therefore the disease may go undiagnosed or improperly treated, resulting in long term symptoms such as nerve pain, facial palsy, heart palpitations, arthritis, and potentially even post-treatment lyme disease syndrome if antibiotic treatment proves ineffective (4). While early diagnosis and appropriate treatment can help prevent the development of late-stage and post-treatment Lyme disease, they are not effective 100% of the time. The development of new treatments and vaccines have progressed slowly, but have thus far not proven effective at reducing the spread of the disease (5,6). This blog addresses and consolidates some of the currently available research on Lyme disease, including reasons for its increasing prevalence, post-treatment Lyme disease syndrome, as well as current and upcoming treatments.
In this webinar, Dr. Audrey Dubourg and Dr. Gareth Guenigault explore the value that an organ-on-a-chip approach can bring to drug discovery and development workflows, and how the regulatory landscape is shifting to encourage their widespread adoption. WATCH HERE
What happens when you get bit by a tick?
It is important to clarify that bites from ticks infected with Borrelia burgdorferi do not always result in infection. Typically, a tick must be attached for 36 to 48 hours or more before B. burgdorferi is able to be transmitted, and if it is removed within the first 24 hours the chances of getting Lyme disease are decreased significantly. However, should the tick remain attached long enough to transmit the bacteria, B. burgdorferi will undergo characteristic changes in gene expression that will allow it to adapt and survive within the mammalian host. Many of the early symptoms and tissue damage that occur from Lyme disease, such as the erythema migrans (EM) or “bulls-eye” rash, arise not from excess presence of the bacteria but from the host’s immune response. The bacterium occur at relatively low densities in affected sites, but are still capable of surviving within a mammalian host for several months to years as they possess a variety of pre-established mechanisms enabling them to adapt to the mammalian physiology.
One such mechanism is antigenic variation, in which the antigens of B. burgdorferi exposed to the immune system can change over time, either through epigenetic modification, DNA recombination, or mutually exclusive expression (8). This ability to maintain itself within a mammalian host for such an extended period of time likely evolved to support the tick-vertebrate infection cycle, allowing B. burgdorferi to be passed on to new generations of ticks. Additionally, B. burgdorferi is particularly resistant to oxidative stress and reactive oxygen/nitrogen species (ROS/RNS), and can thereby circumvent these innate immune responses easily. While the Lyme disease pathogenesis is not yet fully characterized, much of what is known is limited to the early stages of B. burgdorferi infection. While this has allowed for effective early antibiotic treatment of Lyme disease, the long term effects of Lyme disease still require further research, and what is currently known is discussed below (6).
Why have ticks and Lyme disease become more prevalent?
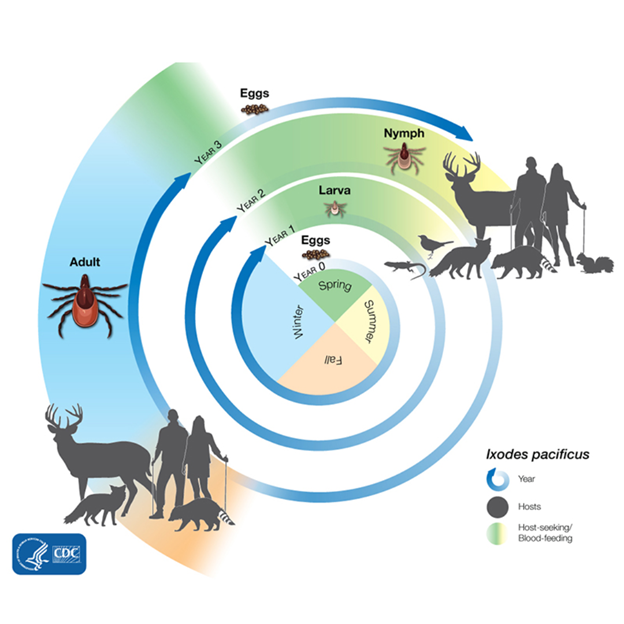
Without an understanding of the tick’s lifecycle and how human development has aided them in their expansion, it can be difficult to grasp why Lyme disease has become so prevalent. While many animals can carry ticks, only a select few act as hosts for this bacteria. White-tailed deer, which are ubiquitous in Canada and the United States, are often thought of as a primary carrier of Lyme disease. However, while they are the primary food source for many tick species, they are not hosts for the bacteria; instead it is small rodents such as the white-footed mouse and the Eastern chipmunk that act as Lyme disease reservoirs. Other mammals and birds still act as hosts for ticks, but contribute far less to Lyme disease prevalence and transmission (2).
Small rodents like the Eastern chipmunk and the white-footed mouse occur frequently in human disrupted environments, such as along forest edges or within small pockets of forest that are often nearby residential areas. These small forests do not provide enough habitat or resources for rodent predators or competitors that could control these chipmunk and mice populations, allowing for unregulated population growth of both ticks and their hosts (2). This is not a new discovery; a 2003 study observing the effects of forest fragmentation on Lyme disease risk found that the smallest forest fragments (less than 1.2 hectares) had the highest proportion of infected nymphs, the second life stage of ticks (9).
Figure 1. Tick life cycle. Lyme disease (Borrelia burgdorferi) is not spread from parent tick to egg: larval, nymph, or adult stage ticks must contract B. burgdorferi from a host. Source: CDC. Reference to specific commercial products, manufacturers, companies, or trademarks does not constitute its endorsement or recommendation by the U.S. Government, Department of Health and Human Services, or Centers for Disease Control and Prevention.
These tick population increases from forest fragmentation are only further compounded by the increasingly warm winters experienced across North America. As tick’s must survive through 2 winters to reach egg-laying adulthood, warmer, milder winters allow for more nymph and larval stage ticks to survive into the next season. This pattern of warmer winters, less predators to control host populations, and increased forest fragmentation near human development has resulted in an upward trend in tick populations worldwide, and subsequently an increase in the prevalence of Lyme disease (2,9).
What is chronic or long-term Lyme disease?
If early antibiotic treatment is not administered, long term manifestations of Lyme disease will develop as the bacteria is transmitted throughout the host. Regional tick variants tend to result in different long term sequelae, however there are three main symptoms of long-term Lyme disease that can affect the nervous system, the heart, and even the joints (4,10).
Neurologic Lyme disease, while fairly rare, comprises about 15% of cases of Lyme disease reported to the CDC, and occurs once the bacterium has reached the peripheral and cranial nerves, or the central nervous systems. Pinched nerves (radiculoneuropathy), facial palsy, and Lyme meningitis are all possible symptoms of late-stage Lyme disease and can be treated with oral or intravenous antibiotics with moderate success. However, permanent nerve damage may result if the disease is able to progress to this degree (3). Some of these symptoms may present themselves 3 to 12 weeks after initial infection, but typically arise after several months or years in patients who did not experience the traditional EM rash (4,10).
Similar to neurologic Lyme disease, Lyme carditis occurs after a prolonged infection in which the Borrelia bacteria have reached the heart (3,4). This long-term manifestation typically presents as feelings of light-headedness, shortness of breath, heart palpitations, fainting, as well as other more typical symptoms of long-term Lyme disease such as fever and body aches. Treatment with oral or intravenous antibiotics typically proves effective after one to six weeks, and, depending on the patient’s condition, a temporary pacemaker may be required (reword). Despite this being a relatively rare manifestation, with 1 in 100 cases of Lyme disease reported to the CDC being Lyme carditis, it can be quite fatal if the disease is able to progress to this point. Between 1985 and 2019, 11 cases of fatal Lyme disease were reported worldwide, and therefore while uncommon, symptoms resembling Lyme carditis should not be taken lightly (3,4,10).
Figure 1: H&E staining of murine kidney tissue experiencing acute (CLK) or chronic (UUO) kidney after treatment with a vehicle control, CBGA, CBD or CBGA and CBD. © 2023 Suzuki et al., licensed under CC BY 4.0.
The most common manifestation of long-term Lyme disease is Lyme arthritis in which the Borrelia bacteria enter the joint tissue and cause inflammation. This occurs in approximately 1 in 4 Lyme disease cases reported to the CDC, but due to reporting practices this may be an overestimation. Inflammation and swelling typically occurs within the knees a few months after initial infection, but can also affect other joints like the shoulder, ankle, elbow, jaw, wrist, and hip. Additionally, this inflammation and movement pain can come and go or even move between joints, making it difficult to determine the root cause of infection if the initial signs of Lyme disease are not present. To address these symptoms, a four-week course of oral antibiotics is usually effective at eliminating the infection and reducing inflammation, however, a second course may be required if joint pain/inflammation persists. If treatment for Lyme arthritis is not administered promptly, therein lies a risk for the development of permanent joint damage. Even if antibiotics are delivered once arthritis symptoms arise, the pain and swelling can remain after two rounds of antibiotics, which can result in the development of post-treatment Lyme disease syndrome (PTLDS), also known as post-Lyme disease syndrome (PLDS) (3,4,10).
What is Post-treatment Lyme disease?
While the overall cause of PTLDS is unknown, a few hypotheses have emerged to explain its prevalence after antibiotic treatment of B. burgdorferi, and are hotly debated among Lyme disease researchers. One such theory is that humans act as Lyme disease reservoirs, maintaining populations of the spirochetes for months to years and supporting the mammal-to-tick infection cycle. However, in a xenodiagnostic study in which uninfected larval ticks were exposed to infected humans with varying stages of Lyme disease, the presence of amplifiable B. Burgdorferi DNA was only detected in two individuals. One had just begun antibiotic therapy and had the characteristic erythema migrans rash, and the other suffered from PTLDS. The authors were unable to culture the spirochete B. burgdorferi bacteria from any individual, indicating that humans likely do not act as reservoir hosts for this disease, or at least do not store the bacteria in their skin as mice do (11). This paper provides support for the counterargument that the symptoms experienced by PTLDS/PLDS patients are due, in part, to an dysregulated host immune response. This is further supported by studies that have found no B. burgdorferi in the joints or surrounding tissues of patients experiencing Lyme arthritis, and the elevated presence of mediators that correlated strongly with autoantibodies to Lyme disease-specific autoantigens (12). While most of the research into this ongoing immune response is preliminary, a handful of studies have demonstrated this prolonged response is at least, in part, due to a dysregulation of host immune responses.
Why isn’t there a Lyme disease vaccine?
While no treatment currently exists that can remedy post-treatment Lyme disease syndrome, preventative vaccinations for B. burgdorferi are close to entering the market. Phase 3 clinical trials began in 2022 for a preventative vaccination known as VLA15, the only Lyme disease vaccine currently in clinical development (13). This vaccine targets the six most common serotypes of outer surface protein A (OspA) of Borrelia burgdorferi, which will prevent the bacteria from being passed to humans from ticks. However, while pre-clinical and clinical studies have demonstrated a strong immune response and a satisfactory safety profile, it is important to consider that the serotypes chosen for this vaccine are most prevalent within Europe and the United States, therefore limiting its worldwide application (13).
A previously available Lyme disease vaccine, known as LYMErix™, was approved by the FDA in 1998. While preclinical and clinical results were promising, a few limitations arose immediately following its approval. Firstly, with a vaccine efficacy of <80%, 20% of vaccinated individuals were still at risk for developing Lyme disease. Additionally, three vaccine doses were required to achieve full protection, with the potential requirement for additional booster vaccinations as often as every year to maintain full immunity. Thirdly, as the clinical trials only evaluated efficacy in people 15 to 70 years of age, its effectiveness in children was uncertain, and this is notable as they are at high risk for Lyme disease. While these initial apprehensions could be addressed with further clinical studies to refine the vaccine, the time it was brought to market was less than ideal as concerns about other childhood vaccines like the mumps, measles, and rubella (MMR) were on the rise.
As initial promotions of the vaccine emphasized the benefits and did not discuss the potential risks, reports of adverse reactions after vaccination began circulating with media outlets putting a human face and story to these side effects. A non-profit citizen action group, known as The Lyme Disease Network, devoted extensive efforts to cover this growing controversy. Side effects varied widely, but frequently mentioned were musculoskeletal complaints such as arthritis, common vaccine side effects such as swelling at the injection site, and systemic symptoms such as myalgias, fever, or chills. The development of autoimmune arthritis appeared to be linked to patients with a specific human leukocyte associated antigen (HLA) type known as the DR4+ genotype. While a study reported that 4 male patients with the DR4+ genotype developed autoimmune arthritis after receiving the LYMErix™ vaccine, the FDA found no association between autoimmune arthritis and the vaccine in question (14).
Between the conflicting scientific literature, pending lawsuits, and questions from the public and media, the FDA reconvened its advisory panel to determine the future of the LYMErix™ vaccine. The panel, described by one participant as raucous and riotous, consisted of FDA scientific advisors, the LYMErix™ manufacturers, independent experts, practicing physicians, as well as the individuals experiencing adverse vaccine effects and their lawyers. Although the physicians demonstrated how the vaccine enabled dramatic reductions in Lyme disease cases, and scientists suggested the potential role of genetic susceptibility and OspA-related autoimmunity, it was clear that the vaccine was contentious, even if it did benefit a wide majority of patients. The panel concluded that the benefits of the LYMErix™ vaccine continued to outweigh its risks, but required manufacturers to provide more vaccine safety and efficacy data in their ongoing phase IV trial. However, after press coverage and ongoing legal troubles, the manufacturer decided to withdraw LYMErix™ in Februrary 2002, citing poor market performance as the primary reason for withdrawing the product. However, it is important to note that a significant proportion of the fear and pushback against the vaccine were derived from the extensive media coverage detailing these moving anecdotal stories of those experiencing adverse vaccine effects, whereas little attention was dedicated to the benefits of preventing Lyme disease. While the practicing physicians who witnessed these benefits firsthand spoke during the advisory panel, their ability to communicate these benefits to patients remained limited as mainstream media focused on the stories of those that experienced the adverse effects (14).
This pattern of early idealization to sudden condemnation was also seen with other vaccines released around this time, such as the RotaShield™ vaccine and other early childhood vaccinations like the mumps-measles-rubella (MMR) vaccine. The fear mongering surrounding vaccinations significantly eroded the public’s trust in childhood vaccinations programmes, an effect still observed at the time of writing. Perhaps if the LYMErix™ vaccine had been released ten years earlier or later, the media and the public may have been more receptive tof the benefits of the vaccines, and less inclined to focus on the risks (14)
Conclusions and Best Practices
With the prevalence of Lyme disease on the rise for the various reasons outlined here, there is understandably growing public interest in the pathology, clinical consequences, and treatment options available. Although the release to market of the previous Lyme disease vaccine was surrounded by controversy, ultimately resulting in short-lived availability, there is cause for optimism. With a new vaccine approaching approval stages in the relatively near future, a new opportunity to learn from, and indeed avoid, past mistakes has been presented. Further, with additional research on the immunological impacts of long-term Lyme disease and post-treatment Lyme disease, a potential cure to address both the preliminary and long-term side effects may also emerge. For now, the best approach remains to follow the advice of public health professionals, namely ensuring long pants are tucked into long socks, and to check for ticks after being outdoors.
For a summary of recommended tick prevention approaches, consult your public health authority, or learn more about the best practices for tick prevention here.
About the Author
About the Author

Jasmin Skinner is an undergraduate student at the University of Western Ontario completing a Specialization in Biology and a Minor in Chemistry, with focused interest in applying these concepts to environmental conservation. As a lover of the outdoors and the arts, much of her time is spent in nature and within the local London art community, creating and connecting with all walks of life. After graduating, she hopes to continue her passion of finding unconventional solutions to environmental issues by working with nature, not against it.
References
- How many people get Lyme disease? [Internet]. [place unknown]: Centers for Disease Control and Prevention; [updated 2021 Jan; cited 2023 Jun]. Available from: https://www.cdc.gov/lyme/stats/humancases.html
- Chobrak U. Why Lyme disease and other tick-borne diseases are on the rise [Internet]. Reno (NV): PBS News Hour; 2022 [cited 2023 June]. Available from: https://www.pbs.org/newshour/science/why-lyme-and-other-tick-borne-diseases-are-on-the-rise#:~:text=Climate%20change%2C%20land%20development%20and,temperatures%20like%20 mammals%20and%20 birds
- Diseases transmitted by ticks [Internet]. [place unknown]: Centers for Disease Control and Prevention; [updated 2022 Mar; cited 2023 Jun]. Available from: https://www.cdc.gov/ticks/diseases/index.html
- Signs and symptoms [Internet]. [place unknown]: Centers for Disease Control and Prevention; [updated 2021 Jan; cited 2023 Jun]. Available from: https://www.cdc.gov/lyme/signs_symptoms/index.html
- Skar GL, Simonsen KA. Lyme Disease. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2023 Jan [updated 2023 May; cited 2023 June] Available from: https://www.ncbi.nlm.nih.gov/books/NBK431066/#:~:text=Like%20syphilis%2C%20Lyme%20is%20classified,12%20months%20of%20the%20infection
- Treatment of Lyme Disease [Internet]. [place unknown]: Centers for Disease Control and Prevention; [updated 2022 Mar; cited 2023 Jun]. Available from: https://www.cdc.gov/lyme/treatment/index.html
- Coburn J, Garcia B, Hu LT, et al. Lyme disease pathogenesis. Curr Issues Mol Biol. 2021;42(1):473-518. doi: 10.21775/cimb.042.473
- Dietsch KW, Lukehart SA, Stringer JR. Common strategies for antigenic variation by bacterial, fungal, and protozoan pathogens. Nat Rev Microbiol. 2009;7(7):493-503. doi: 10.1038/nrmicro2145
- Allan BF, Keesing F, Ostfeld RS. Effect of forest fragmentation on Lyme disease risk. Conserv Biol. 2003;17(1):267-72. doi: 10.1046/j.1523-1739.2003.01260.x
- Mac S, Bahia S, Simbulan F, et al. Long-term sequelae and health-related quality of life associated with Lyme disease: a systematic review. Clin Infect Dis. 2020;71(2):440-452. doi: 10.1093/cid/ciz1158
Ścieszka J, Dąbek J, Cieślik P. Post-Lyme disease syndrome. Reumatologia. 2015;53(1):46-48. doi: 10.5114/reum.2015.50557 - Rebman AW, Aucott JN. Post-treatment Lyme disease as a model for persistent symptoms in Lyme disease. Front Med (Lausanne). 2020;7:57. doi: 10.3389/fmed.2020.00057
- Pfizer and Valneva Initiate Phase 3 Study of Lyme Disease Vaccine Candidate VLA15 [Internet]. [place unknown: Pfizer Inc.]; 2022 Aug [cited 2023 June]. Available from: https://www.pfizer.com/news/press-release/press-release-detail/pfizer-and-valneva-initiate-phase-3-study-lyme-disease.
- Nigrovic LE, Thompson KM. The Lyme vaccine: a cautionary tale. Epidemiol Infect. 2007 Jan;135(1):1-8. doi: 10.1017/S0950268806007096