Over the past decade, there has been increasing interest in understanding the relationship between pharmacokinetic-pharmacodynamic (PK/PD) models and anti-tumor efficacy profiles for putative therapeutic compounds.
Highlights
- Traditional methods used for developing PK/PD models
- PK/PD models and the limitations of traditional dosing methods
- Methods and benefits of implantable microinfusion pumps in both rats and mice
- Validation studies using implantable pumps
- Experimental application of implantable pumps
- The use of implantable pumps to assess TI
- Potential future applications and considerations
Webinar Summary
Taking into account best practices and the 3Rs of animal research (Reduction, Refinement and Replacement), implantable pumps are one of the most effective ways to deliver drugs without interfering with normal animal activity, especially in a group-housed environment. Long-term, chronic dosing regimens and quantitative pharmacology for PK/PD may be scheduled and executed with minimal human intervention and animal stress, and in doing so reduce the impact of these important confounding factors, decrease data variability, and reduce animal use.
In the first portion of this webinar, Christian Schnell began by describing traditional methods used for developing PK/PD models, including the role of PK/PD modeling in oncology research, and the monitoring of plasma concentrations and PD biomarkers over time.
“One of the primary objectives of an oncology drug discovery program is to understand the target coverage required for desired anti-tumor therapeutic efficacy.”
The use of PK/PD data to evaluate their efficacy relationship was demonstrated, including determination of plasma levels required to achieve target levels of biomarker inhibition and the time period after dosing in which desired therapeutic efficacy is expected. The challenge of achieving tumor regression as compared to stasis was highlighted by measuring tumor growth over time in different dose treatment groups and describing the relationship between tumor growth inhibition and effective therapeutic dose duration.
“. . . there is a clear difference between stasis and regression . . . it is a very big hurdle . . .”
A summary of PK/PD models was then presented, including how to elucidate the mechanism of action and better predict the effective dose range in early clinical testing, followed by a discussion of the limitations of this approach such as large animal number requirements. Alternatives to traditional dosing methods were then suggested, specifically parenteral drug delivery, as a means to limit absorption variability, decrease temporal delay, and reduce animal use.
“. . . parenteral drug-delivery technology . . . facilitates validation of novel targets . . .”
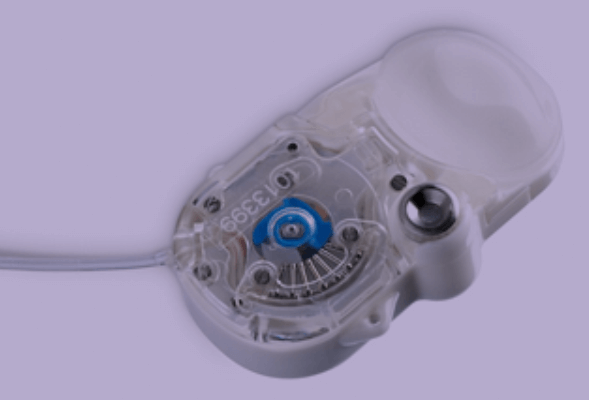
In the second portion of the webinar, the recently developed, implantable, programmable, and refillable SMP-200 iPRECIO pump for continuous infusion was introduced, including a method of catheterization and implantation for use in rats. The SMP-310R pump was also described; this model allows for post-implantation programming during an experiment, and a method for catheterization and implantation in mice was presented. A series of validation experiments were then outlined, including infusion rate assessment, measurement of predicted and observed blood levels, dose and infusion rate variability, and comparison with an osmotic pump. A summary of the validation data was shown, demonstrating a strong correlation between observed and predicted blood level in both rats and mice.
An experimental application of the iPRECIO pumps was described, including measurement of compound plasma levels and tumor growth inhibition in rats and subsequent correlation with tumor PD inhibition, demonstrating that anti-tumor activity was driven by the effective dose duration and not by maximum dose concentration. Replicating this experiment in mice yielded the same result.
“One of the key . . . hurdles . . . we have in oncology is not only to get a compound that is active, but most importantly a compound which is tolerated.”
In the third portion of the webinar the therapeutic index (TI) was discussed, as well as the importance of maintaining clinical efficacy while minimizing toxicity. This included consideration of the limitations of traditional dosing such as higher dose requirements, the need for multiple doses, and short-lived therapeutic ranges with associated side effects. In contrast, pump delivery allows for stable maintenance of drug concentration in the therapeutic range without large fluctuations. Experiments designed to assess TI were then introduced, in which body weight was used as a surrogate marker for toxicity in response to increases in dose with continued PK data collection. Additional data were then presented, obtained using a compound with the known side effect of hyperglycemia, allowing correlation of blood glucose levels with PK to determine appropriate dosing, and comparison with compound plasma levels to calculate TI.
The final portion of the webinar began with a consideration of future directions and the potential to integrate different approaches and technology, such as using pumps in combination with radio telemetry for continuous monitoring. This may allow for tuning of the infusion rate depending on outcome markers, without direct intervention or even remotely (using SMP-310R pumps, for example) in group-housed animals. The limitations of parenteral administration were then discussed, including the need to rapidly achieve steady state concentrations, solubility and stability requirements at normal body temperatures, species-specific restrictions on infusion rate and duration, and battery life considerations.
“. . . parenteral delivery via implantable pumps allows . . . unprecedented PK/PD correlations and corresponding anti-tumor activity . . . without interfering with normal activity in group-housed animals . . .”
The webinar concluded with a summary of the successful and extensive validation study results, and a description of the potential for simultaneous use of complementary novel technologies within the same animal (such as radio-telemetry and programmable pumps as described here) to enable advances in the understanding of complex physiological regulation mechanisms following pharmacological intervention, with the ultimate goal of improving clinical therapies.
Resources
Q&A
- What is the recommended surgery recovery time?
- Do high viscosity formulations work well with iPRECIO pumps?
- Do low solubility compounds and complex formulations work well with iPRECIO pumps?
- Can iPRECIO pumps be combined with telemetry and implanted glucose probes?
- Where should the pump be implanted to reduce or avoid impairment of the animal’s locomotion or welfare?
- Can iPRECIO pumps be used to directly administer compounds to tumors, for example with brain tumors?
- Can you use an initial loading dose to reach steady state faster?
- Are iPRECIO pumps compatible with in vivo imaging systems?
- Can iPRECIO pumps be used for intraocular administration in non-rodent species for glaucoma, AMD treatments, etc.?
- Have iPRECIO pumps been used in larger animals?
- Can iPRECIO pumps be used for intraperitoneal dosing?
- Does pump implantation result in any behavioral changes such as altered locomotion or rearing?
To retrieve a PDF copy of the presentation, click on the link below the slide player. From this page, click on the “Download” link to retrieve the file.
Presenters
Associate Director Oncology NIBR
Novartis in Basel