In this webinar, Dr. Christopher Axelrod and Professor Sander Kooijman introduce the negative health effects of obesity and discuss therapeutic approaches to mitigate them.
Highlights
- Behavioral, surgical, and pharmacological approaches to treating obesity
- Safety, efficacy, and mechanism of action of a mitochondrial uncoupler for weight loss
- Circadian regulation of energy metabolism
- Effects of circadian rhythm disruption on brown fat activity and body weight
Webinar Summary
The global prevalence of obesity increases by approximately 78 million people annually, and more than $2 billion is spent each year to address both medical and societal consequences of this disease. In addition to excess weight gain, obesity is a primary factor for the development of many other diseases such as type 2 diabetes, hypertension, sleep apnea, certain cancers, and cardiovascular disease (CVD). Dr. Axelrod begins this webinar by focusing on three overarching paradigms for creating sustainable weight loss to mitigate the negative health effects and costs of obesity.
“Unlike other diseases, patients with obesity encounter intense stigmatization where societal blame is placed on the person, not the disease.”
Due to the stigma surrounding obesity, many patients do not receive adequate medical treatment, often by choice; for those who do want to receive treatment, options are limited. Behavioral, surgical, and pharmacological treatments against obesity aim to create an energy imbalance to not only reduce body weight, but maintain weight loss in the long-term as well. Behavioral treatments are typically comprised of exercise and calorie restriction, although adherence to these interventions is low, and relapse is common. Surgical treatments are considered the gold standard for effective weight loss, which is sustainable for at least ten years post-surgery. However, many patients are ineligible for weight loss surgeries, and less than 1% of those who are eligible elect to have surgery due to the risk of complications.
Oral and injectable pharmacological treatments act by altering satiety, appetite, and nutrient absorption, but there are a limited number of weight loss drugs available. The four medications currently approved for weight management can cause severe gastrointestinal discomfort and have limited efficacy, failing to produce the weight loss required to improve quality of life in patients with moderate to severe obesity. Furthermore, combination with behavioral and/or surgical interventions is usually required to achieve the degree of weight loss needed for health benefits.
“There is a critical need for medications that at minimum manage obesity-related disease or at best can reverse the causes and consequences of obesity.”
The major limitation of pharmacological therapies that reduce energy intake is that metabolic adaptation occurs over time, which necessitates greater suppression of energy intake. The pharmacological focus of Dr. Axelrod’s talk is on mitochondrial uncoupling agents that work by restricting energy efficiency. However, most prototype uncouplers have narrow therapeutic windows and off-target effects such as depolarization of the plasma membrane, thus limiting pharmacological development in their native forms. Dr. Axelrod is particularly interested in the molecule N5,N6-bis(2-fluorophenyl)[1,2,5]oxadiazolo[3,4-b]pyrazine-5,6-diamine (BAM15), which selectively accumulates in mitochondria and has been shown to have potent uncoupling activity in vitro. The data presented in this webinar address the safety and tolerability of BAM15 in vivo, its efficacy at preventing weight gain, and its mechanism of action.
BAM15 is orally available, safe, and accumulates in lipid-rich tissues, which is a desirable feature of an obesity drug. Using a mouse model, Dr. Axelrod demonstrated that both oral administration and intraperitoneal injection of BAM15 did not alter body temperature at any dose, which is essential for safe delivery. It was also shown that BAM15 primarily distributes into adipose tissue with no negative impact on organ structure or function in other key tissues. To assess the efficacy of BAM15, mice were placed on a 60% high fat diet (HFD) to induce obesity and received either placebo or 0.1% BAM15 treatment, or were calorie-restricted. While both calorie-restricted and BAM15-treated mice were protected from diet-induced obesity compared to control animals, weight loss maintenance was only observed in BAM15-treated mice. BAM15 also enhanced glycemic control independent of body weight compared to calorie restriction, demonstrating that BAM15 improves obesity in a way that is not strictly a function of weight loss.
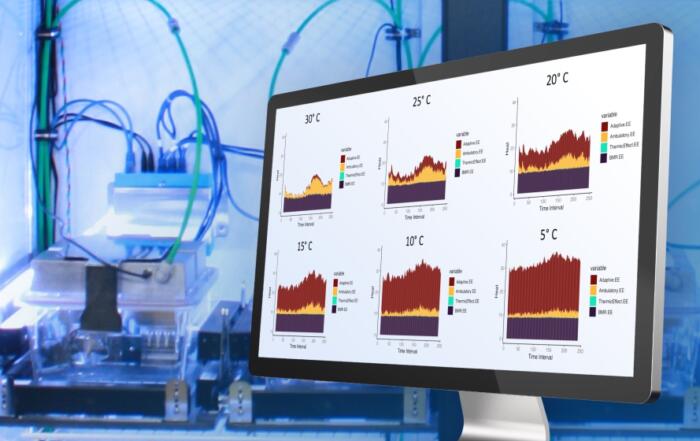
Lastly, the mechanisms by which BAM15 reduces body weight and improves glycemic control were assessed by evaluating energy expenditure and substrate utilization using a Promethion system. Despite no change in body temperature, BAM15-treated mice exhibited a significant increase in daily energy expenditure compared to control animals, as well as a lower respiratory quotient which is indicative of whole-body fat utilization. Dr. Axelrod also sought to identify signaling nodes that link uncoupling-mediated processes to changes in substrate utilization. RNA sequencing revealed that BAM15 activates adenosine monophosphate-activated protein kinase by direct uncoupling and indirect stimulation of the insulin receptor signaling pathway. BAM15 also exhibited no effect on lipogenesis in any white adipose tissue depot, but did increase skeletal muscle oxidation of fatty acids. Dr. Axelrod concludes that BAM15 likely inhibits adipogenesis rather than enhances lipogenesis, and is currently working towards understanding how bioenergetic efficiency may regulate lipogenic processes.
In the second half of this webinar, Professor Kooijman focuses on obesity in the context of CVD, as well as circadian regulation of energy metabolism. Since the human body is naturally active during the day and inactive during the night, energy demands must be adapted accordingly. Consequently, most metabolic processes are under circadian rhythm control, primarily done by light exposure. Any small disruption in light exposure or other factors such as physical activity, food intake, temperature, and hormones affect the likelihood of CVD development, which Professor Kooijman notes is particularly true for shift workers, who are at a higher risk for obesity, diabetes, and CVD than the general population.
“The problem with these diseases is that they are chronic and progressive, and so it can be both difficult and time-consuming to study these associations in humans, let alone the effect of interventions, and for that reason we … need animal models.”
Brown adipose tissue (BAT) uses lipids and glucose for heat production, and is primarily active during periods of sleep and cold winter months. Since BAT takes up glucose and lipids, radio-labeling can be used to monitor its activity in both humans and mice. Professor Kooijman presents a study of a well-established mouse model for human-like lipoprotein metabolism; these mice were injected with a β3-adrenergic receptor antagonist to protect from atherosclerosis, which can lead to CVD. Rapid clearance of lipids from circulation into BAT depots was observed by radio-labeling in the treatment group. In addition to decreased plasma triglyceride levels, BAT activation and fatty acid uptake resulted in decreased cholesterol since delipidated lipoproteins are more prone to uptake by the liver. A direct consequence of decreased triglyceride and cholesterol levels is decreased atherosclerotic plaque formation, suggesting that CVD can be prevented by BAT activation.
Further radio-labeling studies revealed that BAT activity in mice is characterized by circadian, diurnal, and circannual rhythms, with mice gaining more fat mass during prolonged light exposure conditions. Monitoring mice during short (8 hours), normal (12 hours), and prolonged (16 hours) light exposure revealed a pronounced rhythm in fatty acid uptake by BAT that corresponded to the light cycle. Furthermore, under summer-like prolonged light conditions, uptake of fatty acids by BAT was reduced compared to short light conditions.
“It’s very tempting to speculate that this mechanism prepares the body for temperature changes … with seasons.”
The consequences of eating at abnormal times, which happens during shift work, were also assessed in a mouse model; mice were either exposed to weekly changes in their light cycle or six hour advances or delays in light exposure each day. Atherosclerosis development was observed in mice whose circadian rhythms were disrupted; more extreme shifts in the light cycle resulted in higher atherosclerotic lesion area and severity. Surprisingly, no effect was observed on BAT function, plasma lipid levels, weight gain, or circulating immune cell number or activation status. The effect of eating on plasma lipid levels was also assessed in humans by providing volunteers with three isocaloric meals. Lipids were found to accumulate more in the blood during lunch and dinner rather than during breakfast, which is expected since BAT in humans is thought to be most active at times of wakening.
“This old saying, “eat breakfast like a king, lunch like a prince, and dinner like a pauper” may be true.”
Lastly, the Kooijman group evaluated sex differences in the response to modeled shift work by comparing the effects of simulated jet lag on male and female mice. Attenuated voluntary activity and weight gain in response to circadian disruption were only observed in jet-lagged male mice. Plasma glucose levels were not affected by jet lag in male mice, but plasma insulin levels were increased, suggesting there is some form of insulin resistance. In contrast, increased plasma glucose and triglyceride levels were observed in jet-lagged female mice. Circadian disruption therefore differentially affects glucose homeostasis in male and female mice. Furthermore, the misalignment between food intake and the body’s ability to process nutrients results in triglyceride and glucose accumulation in the circulation. In male mice, these triglycerides are taken up by BAT depots, leading to fat mass gain and ectopic fat deposition. In female mice, triglycerides and glucose remain in circulation, posing a risk for protein glycosylation, endothelial dysfunction, oxidative stress, local inflammation, and insulin resistance that could lead to atherosclerosis development.
Outstanding questions that Professor Kooijman hopes to answer in future studies include how to prevent cardiometabolic problems caused by circadian disruption and whether circadian rhythms must be considered when measuring metabolic activity. Professor Kooijman also hopes to maximize the effectiveness of chronotherapy by understanding the circadian regulation of energy metabolism. The effects of cold on energy expenditure in mice can be masked by changes in physical activity, and so mice may not be the best models to study the effects of cold exposure timing on energy metabolism. Preliminary data in humans show that cold exposure in the early morning leads to an increase in energy expenditure, but not at the end of the day. Therefore, the Kooijman group plans to determine whether circadian cycles in metabolic tissues should be considered during chronotherapy.
Resources

Q&A
- Have studies been conducted to determine the long-term safety and efficacy of BAM15?
- Are female shift workers more susceptible to CVD than male shift workers?
- Might BAM15 treatment alter the fatty acid composition of the body?
- Are there any breathing test data that show the rates at which animals are oxidizing endogenous lipids?
- Did BAM15 alter BAT function or thermogenesis?
- Have you measured energy expenditure under thermoneutral conditions?
- Do you plan to take this research into humans?
To retrieve a PDF copy of the presentation, click on the link below the slide player. From this page, click on the “Download” link to retrieve the file.
Presenters
Director of Translational Services
Integrated Physiology and Molecular Medicine Laboratory
Pennington Biomedical Research Center
Assistant Professor
Endocrinology
Leiden University Medical Center