Protocol Preview: In Vivo Gene Therapy Using Peptide-Based Delivery
Blog post by Jasmin Skinner
Gene therapy has been in discussion since 1972, but its translation to clinical practice has been a slow and challenging process [1]. Aside from its steep cost at 1.6 million dollars per patient [2], current gene modification methods are ethically questionable, short-lived, and can result in harmful immune responses [3]. More serious complications can also arise due to off-target effects, such as germ line mutations or activation/alteration of the incorrect portion of the genome [3]. Although current in vivo gene therapies are ineffective and controversial, a novel peptide-based delivery method could drastically improve future studies.
Current problems with in vivo gene therapy
While many researchers boast the benefits of gene therapy for treating recessive genetic disorders (e.g., sickle cell anemia, cystic fibrosis), very few therapies have seen success in clinical trials, in part due to the choice of vector [3]. Since viruses (and viral vectors) have evolved over millions of years to deliver DNA and RNA into a foreign host, they are extremely effective at delivering genetic material to a target cell in the context of gene therapy [4]. However, viral vectors must first get past the immune system. Immunity to viral capsules is one of the biggest challenges in gene therapy to date, as commonly used vectors are derived from harmless viruses circulating in humans [4]. Additionally, an undesired immune response could occur and be fatal for immunocompromised individuals [4].
On the other end of the spectrum, non-viral vectors for in vivo gene therapy greatly reduce the risk of unwanted immune responses, but with the trade-off of lower delivery efficiency [5]. Although advances in specificity, efficiency, and gene expression duration have led to their increased use in clinical trials, non-viral vector properties can still be improved [5]. As a solution to this translational challenge, Allen et al. have proposed a peptide-based delivery method of Cre recombinase for in vivo gene therapy [6].
In this webinar, Marta Walasek, PhD, and Danielle Nguyen Truong, BSc, provide an overview of hematopoietic stem and progenitor cells (HSPCs) and CRISPR-Cas9 genome editing, and define optimal culture conditions for an efficient genome editing workflow. WATCH NOW
Benefits of a peptide-based delivery method for gene modification
The authors sought to compare the efficacy of this peptide-based delivery method to adeno-associated viruses (AAVs), the most common in vivo gene delivery technique used to date. While AAVs are non-pathogenic and predictable, they have a very limited packaging capacity, slow gene expression, low transduction efficiency, and the potential to incorporate themselves into the host genome. Direct protein delivery can alleviate some of these issues, as intracellular activities can be initiated without a delay in transcription or translation. However, the technology for facilitating this direct delivery must first be improved, which the authors addressed in the present study.
By utilizing dfTAT peptides that selectively permeabilize late endosome membranes, a protein payload can be endocytosed and released into the cytoplasm. This peptide-based protein delivery method has been tested in vitro, but not in vivo where the peptide and protein payload could diffuse away from each other. To assess its efficacy, the authors stereotactically injected the peptide protein payload into mice intracranially and compared it to standard AAV transduction.
Study parameters and methodology
This novel in vivo gene therapy protocol begins with solid-phase peptide synthesis of dfTAT, a fluorescently-labeled dimer of the cell-penetrating peptide TAT, which can efficiently deliver small molecules, peptides, antibodies, and biologically-active enzymes to the cytosol of cells (Figure 1). Following peptide synthesis, the TAT-Cre recombinase gene was transfected into Escherichia coli cells, which then underwent a series of inoculations and inductions to create a concentrated solution. These cells were then pelleted, lysed, and centrifuged to remove cell debris, after which TAT-Cre was purified via metal affinity chromatography. An AAV2-Cre complex was also created for comparison.
Male and female mice were anesthetized and placed on a stereotaxic device, after which small volumes of peptide-protein payload or AAV2-Cre mixture were injected into the sensorimotor region of their cerebral cortex over a duration of five minutes. The mice were euthanized two weeks after this surgery; semiserial coronal sections of their brains were cut, centered around the injection site. Multiple staining methods were used to determine which cells exhibited the appropriate gene expression, and the remaining intact brain slices were imaged to analyze cell density.
Is a peptide-based delivery effective for in vivo gene therapy?
Following delivery of Cre recombinase, a rapid onset of intracellular biological activity occurred as the enzyme was delivered in its active form. The enzyme was shown to be directly and transiently delivered into target cells. Additionally, the enzyme did not cause off-target or knock-on effects that often occur with prolonged exposure.
Implications, limitations, and future directions
This peptide-based delivery method for in vivo gene therapy was shown to be as effective as AAV gene delivery, the current standard for genetic modification. While this approach is not meant to replace current AAV protocols, it can provide researchers with an alternative approach for delivering in vivo gene therapies. Peptide-based protein delivery has many advantages over its predecessors, including direct payload delivery, reduced production time and costs, and no risk of DNA integration with the host genome. However, this methodology does not completely address all of the concerns associated with in vivo gene therapy, and will require additional testing before it is ready for clinical use. Nevertheless, peptide-based protein delivery does provide an exciting opportunity for the advancement of in vivo gene therapy, and could identify new pathways for genetic modification.
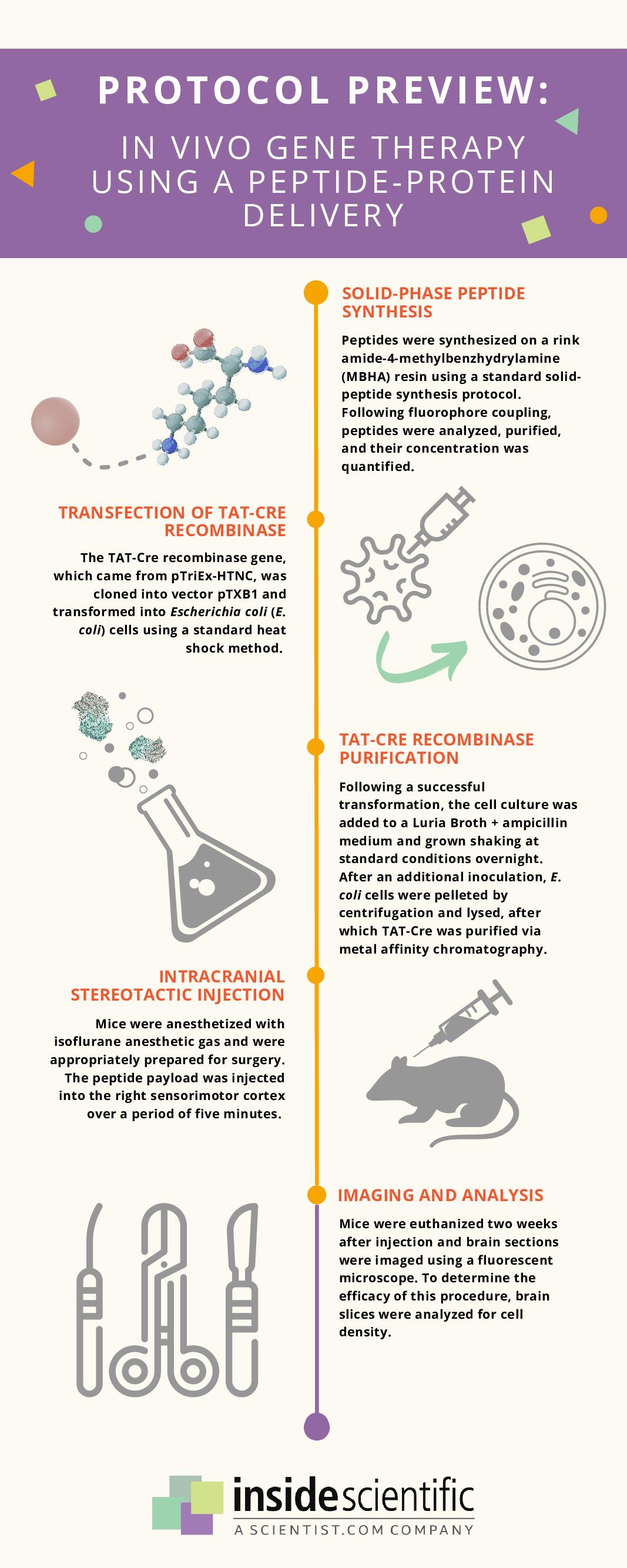
Figure 1: Illustration of the methodology for a novel peptide-protein delivery technique for in vivo gene therapy. Adapted from ref. 6.
About the Author
About the Author

Jasmin Skinner is an undergraduate student at the University of Western Ontario completing a Specialization in Biology and a Minor in Chemistry, with focused interest in applying these concepts to environmental conservation. As a lover of the outdoors and the arts, much of her time is spent in nature and within the local London art community, creating and connecting with all walks of life. After graduating, she hopes to continue her passion of finding unconventional solutions to environmental issues by working with nature, not against it.
References
- Friedmann T, Roblin R. Gene therapy for human genetic disease? Science. 1972;175(4025):949-55. DOI: 10.1126/science.175.4025.949.
- Bioentrepreneur. $1-million price tag set for Glybera gene therapy [Internet]. Springer Nature. 2019 – [cited 2022 Dec 20]. Available from: www.bioengineeringcommunity.nature.com/posts/45406-1-million-price-tag-set-for-glybera-gene-therapy.
- Gonçalves GAR, de Melo Alves Paiva R. Gene therapy: advances, challenges, perspectives. Einstein (Sao Paulo). 2017;15(3):369-75. DOI: 10.1590/S1679-45082017RB4024.
- Capra E, Godfrey A, Loche A, Smith J. Gene-therapy innovation: unlocking the promise of viral vectors [Internet]. McKinsey & Company. 2021 – [cited 2022 Dec 20]. Available from: www.mckinsey.com/industries/life-sciences/our-insights/gene-therapy-innovation-unlocking-the-promise-of-viral-vectors.
- Ramamoorth M, Narvekar A. Non viral vectors in gene therapy- an overview. J Clin Diagn Res. 2015;9(1):GE01-6. DOI: 10.7860/JCDR/2015/10443.5394.
- Allen JK, Sutherland TC, Prater AR, Geoffroy CG, Pellois JP. In vivo peptide-based delivery of a gene modifying enzyme into cells of the central nervous system. Sci Adv. 2022;8(39):eabo2954. DOI: 10.1126/sciadv.abo2954.
