Q&A Report: Fully Characterized, Standardized Human Induced Pluripotent Stem Cell Line and Ready-to-Use, High-Quality Neural Progenitor Cells for Downstream Differentiation Applications
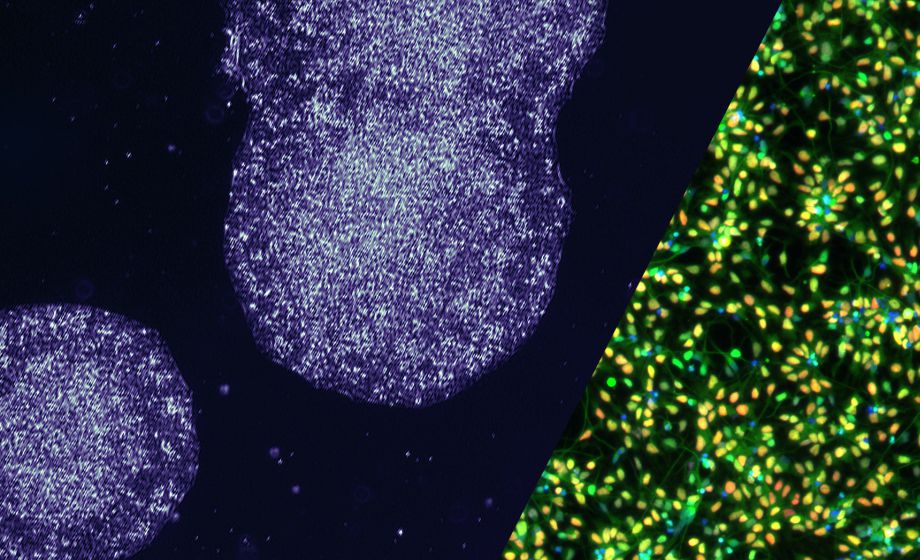
On June 29, 2023, STEMCELL Technologies’ Dr. Andrew Gaffney and Dr. Erin Knock presented on how to streamline PSC-related workflows using highly characterized induced pluripotent stem cells (iPSCs) and neural progenitor cells (NPCs), and how to differentiate these cells into a variety of cell types downstream. In this Q&A report, you can read their answers to questions asked by the audience. Answers have been edited for length and/or clarity.
What is the difference between SCTi003-A and iPSCdirect™?
Dr. Andrew Gaffney: SCTi003-A is a parent cell line that we’ve used to make other products. iPSCdirect™ is manufactured from the SCTi003-A cell line; however, it is a high-density and single-use version of the SCTi003-A cell line. If you procure the SCTi003-A cell line, it is frozen in clumps/aggregates with approximately one million viable cells per vial. In contrast, iPSCdirect™ is provided as a single-cell suspension with approximately ten million viable cells per vial so you can count exactly how many cells you’re placing into each vessel. The choice of which product to use depends on your application: if you want to keep your cells in long-term culture, you’d buy the SCTi003-A cell line, and if you’re looking to immediately initiate downstream experiments, such as differentiation or transfection 24 hours after thawing your cells, we’d recommend using iPSCdirect™.
What do you mean by "single use" for iPSCdirect™?
Dr. Andrew Gaffney: The main difference between the two products, the SCTi003-A and iPSCdirect™, is the format in which we’ve generated the product. SCTi003-A is intended for long-term culture—you can keep the cells going for many passages and pull the cells out of long-term culture whenever you wish to use them. What we mean by single use for iPSCdirect™ is that you simply take a vial out of the freezer whenever you want to use it and immediately perform your downstream experiments. There are also differences in the permitted use and licensing for the two products; long-term culture is not permitted without a license. There is a license fee associated with the SCTi003-A cell line, whereas there is no license fee for iPSCdirect™ because it’s not intended for long-term culture.
How many vials are included in the purchase?
Dr. Andrew Gaffney: One vial of cryopreserved cells is included in each order.
Are there any license fees involved in using SCTi003-A and iPSCdirect™? Does the iPSC-derived NPC have a license fee?
Dr. Andrew Gaffney: There is a fee with the SCTi003-A cell line, which is $5000 USD per year for commercial entities. Non-profit organizations interested in using SCTi003-A may apply for a license fee waiver. There are no fees associated with either iPSCdirect™ or the Human iPSC-Derived Neural Progenitor Cells (NPCs).
What is the difference in passage numbers between SCTi003-A and iPSCdirect™?
Dr. Andrew Gaffney: The SCTi003-A cell line is frozen at passage 32 and iPSCdirect™ is frozen at passage 35.
Are the non-direct SCTi003-A cells frozen as clumps or single cells?
Dr. Andrew Gaffney: We freeze the parent line as clumps or small aggregates at approximately one million viable cells per vial. When you thaw this vial for the first time in your lab, we recommend seeding at a range of densities across the 6-well plate, allowing them to recover for a week, and picking the well with the optimal clump morphology to continue your long-term passages with.
SCTi003-A is a genetically female line. Do you have a plan for a genetically male line?
Dr. Andrew Gaffney: Yes, we do and we hope to make many more lines in the future that are similar in quality standards to the SCTi003-A cell line.
Which is the highest passage for iPSCs until they are not viable anymore? For example, can I start a differentiation protocol with iPSCs in passage 47?
Dr. Andrew Gaffney: One classic feature of iPSCs is that they can be expanded in the undifferentiated state indefinitely. As long as the iPSCs are still expanding in this undifferentiated state, we wouldn’t say that they’re not viable anymore. Differentiation experiments could be initiated at any passage number. One risk is that with human pluripotent stem cells, there is an association between higher passage numbers and links to commonly acquired karyotypic abnormalities, which is why it is important to check the cells fairly frequently. It’s also been noted that some of these abnormalities have been linked to skewed or limited differentiation capacity. We provide the cells at passage 32 and we perform a lot of quality control on these cells; so, after initiating these cultures, these cells would be fine to differentiate straight away. We’ve previously kept them going for around 20 passages so far in-house and they’ve remained karyotypically stable. So, we’d say that as long as our protocols are followed, it should be fine at passage 47, but you should still be checking the quality of your cells.
Do we have the option to culture these iPSCs with any other media besides the recommended mTeSR™ Plus?
Dr. Andrew Gaffney: We developed the SCTi003-A cell line for maintenance in mTeSR™ Plus medium, which is also the medium the cells were expanded in before banking. We do strongly recommend that you recover the iPSCs in the medium in which they were maintained before freezing, i.e. mTeSR™ Plus. Our protocol for thawing states that these cells must be thawed in mTeSR™ Plus, and I would recommend you do that. If you do need to switch media downstream, I don’t see why it wouldn’t work; but we haven’t tried or validated other downstream media ourselves. The only thing that I would stress is that when the cells are thawed for the first time, they should go into mTeSR™ Plus.
Do the cells need to be plated on Matrigel®-coated wells?
Dr. Andrew Gaffney: For the iPSCs, I previously mentioned the culture system during manufacture is mTeSR™ Plus on hESC-qualified Matrigel®. Again, this can be switched downstream, but because it was manufactured in that system we recommend thawing into that system. We have also tried thawing these cells onto Vitronectin XF™ matrix, and that has worked as well. Feel free to get in touch with us if you’re looking for a protocol to thaw these cells on Vitronectin XF™.
Dr. Erin Knock: Our STEMdiff™ SMADi Neural Induction Media protocol is generally done on hESC-qualified Matrigel® as well, but for the Human iPSC-Derived Neural Progenitor Cells (NPCs), if you want to thaw them and expand them, you can do that on Matrigel® or you can do that on PLO-Laminin. For neuronal differentiation, we generally recommend PLO-Laminin or PDL-Laminin-coated plates.
Is it better to generate neurons from cryopreserved NPCs or neuron precursors?
Dr. Erin Knock: I don’t think it’s a question of “better”, I think it’s a question of what you need them to be able to do. The advantage of starting from Human iPSC-Derived Neural Progenitor Cells (NPCs) is that you have a little bit of expansion time before you differentiate into your neurons. If you’re looking to generate a large number of neurons, you can thaw the NPCs, expand them up to get a large number of NPCs, and then differentiate a large number of neurons. Alternatively, if you have some uncertain timelines and want to expand your NPC line and then freeze them down and thaw them again and start later, you can also do that. So NPCs give you that kind of flexibility. Compare this to neuron precursors, which are always already on that path to becoming a neuron; they’re not proliferative anymore and you won’t be able to expand them. You can also freeze them down but they’re not going to be as robust as the NPCs when you freeze and thaw them. This might lead to a small reduction in yield but in terms of purity and efficiency of differentiation, they’re pretty equivalent.
What's the best way to get quality NPCs from fully characterized iPSCs?
Dr. Erin Knock: The best way to get NPCs from iPSCs is by using STEMdiff™ SMADi Neural Induction Media. Two different protocols can be used for neural induction using the STEMdiff™ SMADi Neural Induction Media. There is the embryoid body protocol and the monolayer protocol. We find that the embryoid body protocol is best to start with if you don’t know the neurogenic capacity of your iPSC line. If you are not using the SCTi003-A cell line and are using something else and you don’t know if it’s going to make good quality NPCs or not, the embryoid body protocol is robust—it will get you some neuroectoderm and you’ll have to judge the efficiency based on your starting cell line. Whereas, for a line that you already know has a good neurogenic capacity (i.e. it already differentiates well into the NPCs and neurons, similar to the SCTi003-A cell line), the monolayer protocol is technically simpler. It tends to be more efficient every time, resulting in less technical variability. So for certainty, we lean toward the embryoid body protocol, and for reproducibility, we lean toward the monolayer protocol. You can also just purchase our iPSC-derived NPCs instead of differentiating your own.
What transfection reagent is recommended? Is there a way to know the components of the STEMdiff™ SMADi Neural Induction Media? Are these differentiated cells mobile, and do they migrate when coated on ESC Matrigel®?
Dr. Erin Knock: You can use either an electroporation or lipid-based transfection system to transfect the iPSC cells. We recommend that if you’re plating cells down close to transfection, you do so in CloneR™2 because that will greatly increase the survival of your cells post-transfection. As for the components of the STEMdiff™ SMADi Neural Induction Media, we can’t divulge our formulations but I think it’s pretty obvious in the name that the main driving force behind the media kit is the dual SMAD inhibition. So, based on a lot of the literature looking at PSC-to-neuroectoderm differentiation, we’re targeting a lot of those same pathways with the dual SMAD inhibitors that are present in the kit. Additionally, the basal media is formulated to increase the optimal health and survival of the cells during that PSC-to-NPC transition. For the last question, I assume the mobility is in regards to the NPCs; you’ll see a few of them moving around but they are not migratory by any means. The NPCs will generally stay within the confined area; they might move around a little bit, but as they start differentiating, they will elongate and then remain fixed in place.
In the STEMCELL Technologies protocol, they mentioned that I could passage NPCs up to 10 passages but I observed abnormal morphologies from passage 5. Is that normal?
Dr. Erin Knock: I think it tracks along very well with what I mentioned in the latter part of the webinar where, after passage 5, the NPCs will start to transition from a neurogenic fate to a gliagenic fate. That transition to gliogenesis is often associated with a change in morphology, where you’ll see cells that were very small, phase-dark, and defined, becoming larger and more spread out. There are a wide range of astrocyte morphologies, from something that is very protoplasmic-looking, to something that is very spiky, to radial glia that are neural in morphology. So, when I hear people saying that, after passage 5, I’m seeing something abnormal in morphology, what they are seeing is that range in glial morphology that is starting to show as that transition from primarily neurogenesis to primarily gliogenesis is occurring. You can continue to passage the NPCs beyond passage 5, but it’s just that after passage 5, you’re going to see a shift as the percentage of neurons being generated decreases and the percentage of glia being generated increases.
Once mature astrocytes have been derived, how many passages can I obtain from these?
Dr. Erin Knock: I’ll take this question from two different angles because there are two ways to go about this question. If you are thinking about how many passages of the NPCs you can derive mature astrocytes from, then that’s up to passage 10 so far. If you’re thinking about once you’ve derived the astrocytes, we’ve passaged them up to an additional 10 passages after our STEMdiff™ astrocyte maturation phase, so they can go on quite a ways, but it depends on the cell line you’re using. For example, if you start from the SCTi003-A-derived NPCs and derive astrocytes from them, we’ve found that if you start from later-passage NPCs (due to the switch from neurogenesis to gliogenesis), you’ll get a higher rate of gliogenesis, and the resulting astrocytes can be passaged 10 – 20 times.
Should I start with the iPSCs or the NPCs to make neurons? Are there any advantages to using one over the other?
Dr. Erin Knock: There are protocols out there for both. Assuming that the end goal is to generate neurons from either the iPSCs or NPCs, starting from Human iPSC-Derived Neural Progenitor Cells (NPCs) is faster because they’ve already gone through the neuroectoderm phase. So, you can generally get neurons from NPCs in 3 – 5 days. Starting from iPSCs, such as SCTi003-A, might take a little longer and the usual protocols might take 7 – 10 days. If you start from iPSCs, you’re going to have almost indefinite expansion beforehand whereas with NPCs, after passage 5 you will start seeing a lot more spontaneous differentiation toward the glial lineage, making neuron differentiation less efficient.
Does gene editing interfere with cell differentiation? Do you think that cells edited using CRISPR/Cas9 could be less prone to differentiate?
Dr. Erin Knock: The success of differentiation downstream of CRISPR editing depends on the gene you are editing and the potential off-target edits. If you knock out a gene that is critical for neural differentiation, you will see poor differentiation. Similarly, if you have an off-target edit in a gene that regulates differentiation, you may see poor differentiation. You must assess the pluripotency via tri-lineage differentiation and perform whole genome sequencing as part of your quality control when creating an edited PSC line.
How can we get basal progenitors? And how can we distinguish apical and basal progenitors?
Dr. Erin Knock: In 2D culture, we typically see apical progenitors, because 2D cultures often lack the organization required for basal progenitors to form. Occasionally, if you have a high amount of rosette-like structures forming in your monolayer NPC culture, you can see the presence of basal progenitors. If you want more physiological ratios of apical and basal progenitors, an organoid system will be more efficient.
Is it possible to freeze the NPCs at a lower density than suggested and perform experiments immediately after thawing? With a lower NPC density, can I leave the cells without changing the medium over the weekend?
Dr. Erin Knock: We do tend to find that Human iPSC-Derived Neural Progenitor Cells (NPCs) survive better at higher densities and we recommend keeping them to “>100%” confluence before passaging, meaning that they are starting to multilayer over themselves. If you seed at a lower-than-recommended density, you may see poorer survival. However, if you thaw on a Friday at the recommended density and do a double feed with STEMdiff™ Neural Progenitor Medium, you can probably change the medium again Monday with minimal effects.
Is there any way to mark living NPCs with Doublecortin (DCX) (as you mentioned, the marker of newborn neurons)? I want to track NPC migration, and DCX is an intracellular protein that requires cell permeabilization.
Dr. Erin Knock: There isn’t a good way to stain for DCX in live cultures. We do have NeuroFluor™ NeuO, which will start being expressed after 7 days of differentiation, so it may be a bit late for “newborn” neurons.
During the monolayer protocol for generating NPCs, what should we do when we see a lower density than expected, with "holes" without cells?
Dr. Erin Knock: Holes, in themselves, are not a deal breaker, as long as your remaining cells consist of a high percentage of PAX6- and SOX1-positive cells. To keep the density high and retain good expansion and survival, my recommendation is to keep the cells a day or two past the recommended 6 – 7-day passage time, then passage and re-seed them at a slightly higher density than your previous passage.