Join Diane Ramsden, PhD, and Lindsey Whaley, MS, for an inside look at TruVivo™, a novel all-human in vitro hepatocyte system developed by LifeNet Health LifeSciences.
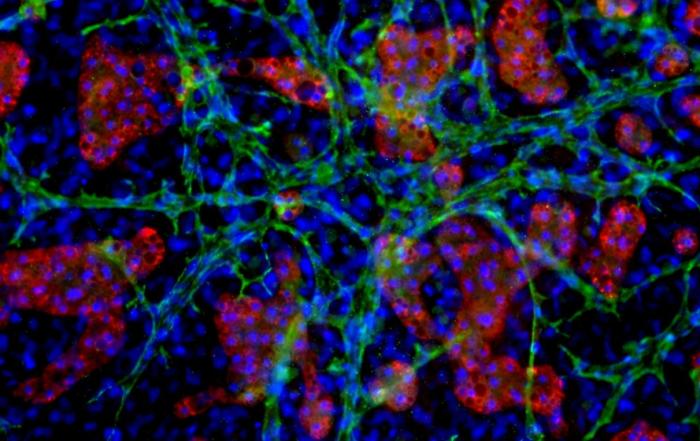
Current in vitro hepatic models, such as sandwich cultures, lose key hepatocellular function and enzymatic activity over time. More advanced co-culture platforms have been introduced but are often complex or contain non-human cells that can alter results due to innate background contribution to metabolism. These limitations make it challenging to characterize human mechanisms for the metabolism of drug candidates, especially those that are slowly metabolized. To address these limitations, TruVivo was developed as the first and only 2D+ hepatic system to provide human-relevant, reliable results in an easy-to-use format.
In this webinar, Lindsey Whaley introduces key features of the TruVivo system that demonstrate its potential to be an ideal platform for drug discovery and development applications. Diane Ramsden then presented data from her evaluation of TruVivo as a potential tool for investigating mechanistic drug metabolism and pharmacokinetics, including drug-drug interaction potential, and translational risk assessment.
Key Topics Include:
- The advantages of the system – which offers the simplicity of a 2D model, with the robust levels of data typical of 3D models.
- An overview of how TruVivo mimics the microarchitecture and functionality of the human liver.
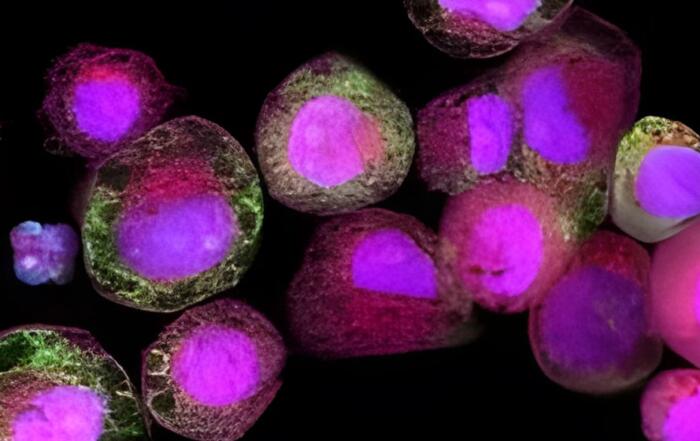
- How hepatocytes are pre-qualified in the system to reduce variability for a broad range of pharmacological and toxicological applications.
- Drug metabolizing enzyme activity and retention over the use period.
- Translation of in vitro derived parameters to conduct DDI risk assessment.
Presenters
Director
Oncology DMPK
AstraZeneca
Product Manager
LifeSciences
LifeNet Health